第三次扩项!中关村水木医疗顺利通过“放疗、网络安全”领域扩项现场评审
2023-12-06 11:27:28source | 网络
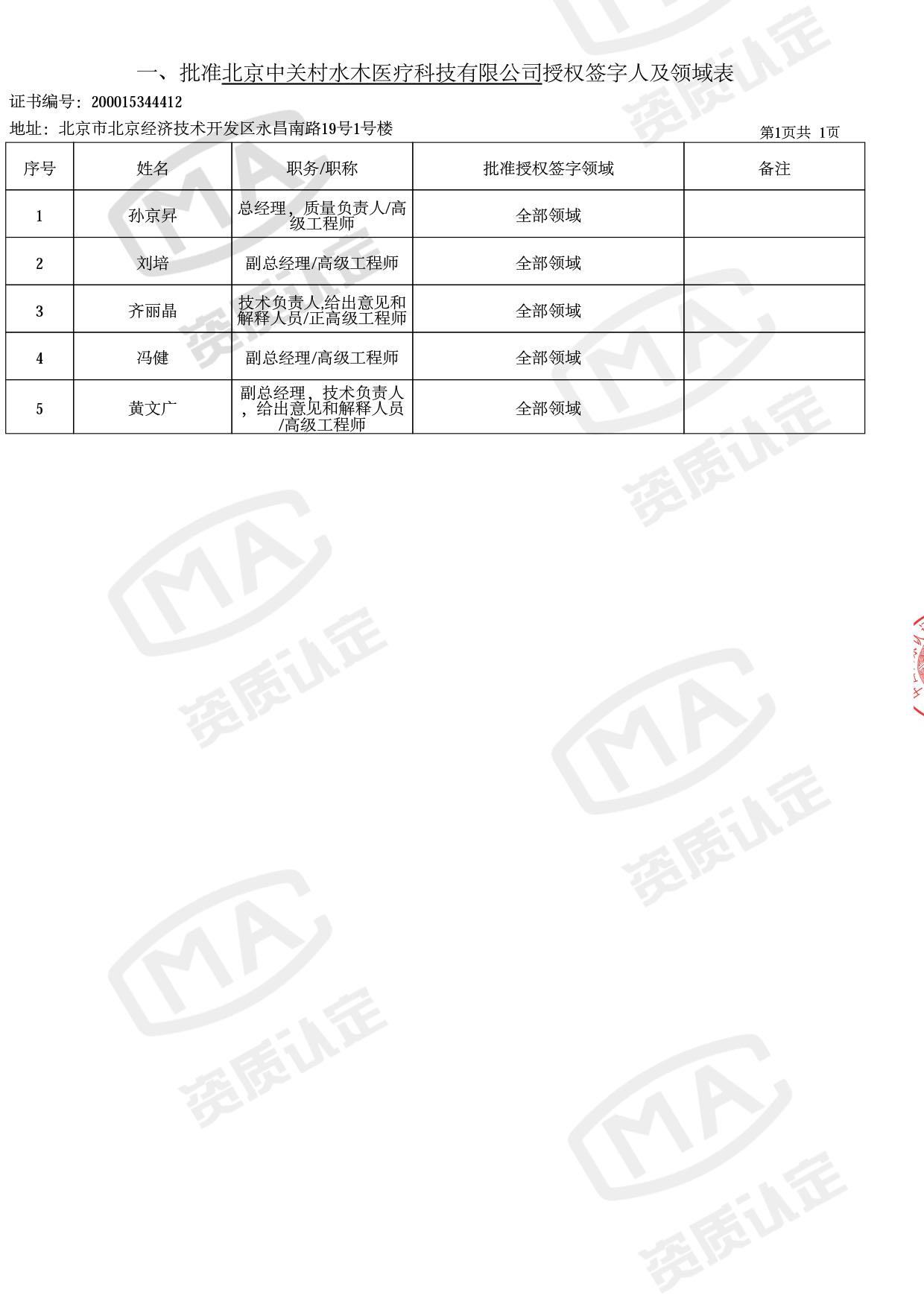
近日,市场监督管理总局委派评审专家,对中关村水木医疗进行现场审核,评审组对公司医疗器械技术领域质量管理体系运行和质量监督等情况进行检查,并对本次扩大范围的5位授权签字人进行现场考核。
经过实验室全体成员的充分准备、积极配合,本次扩项工作圆满完成,成功获批。
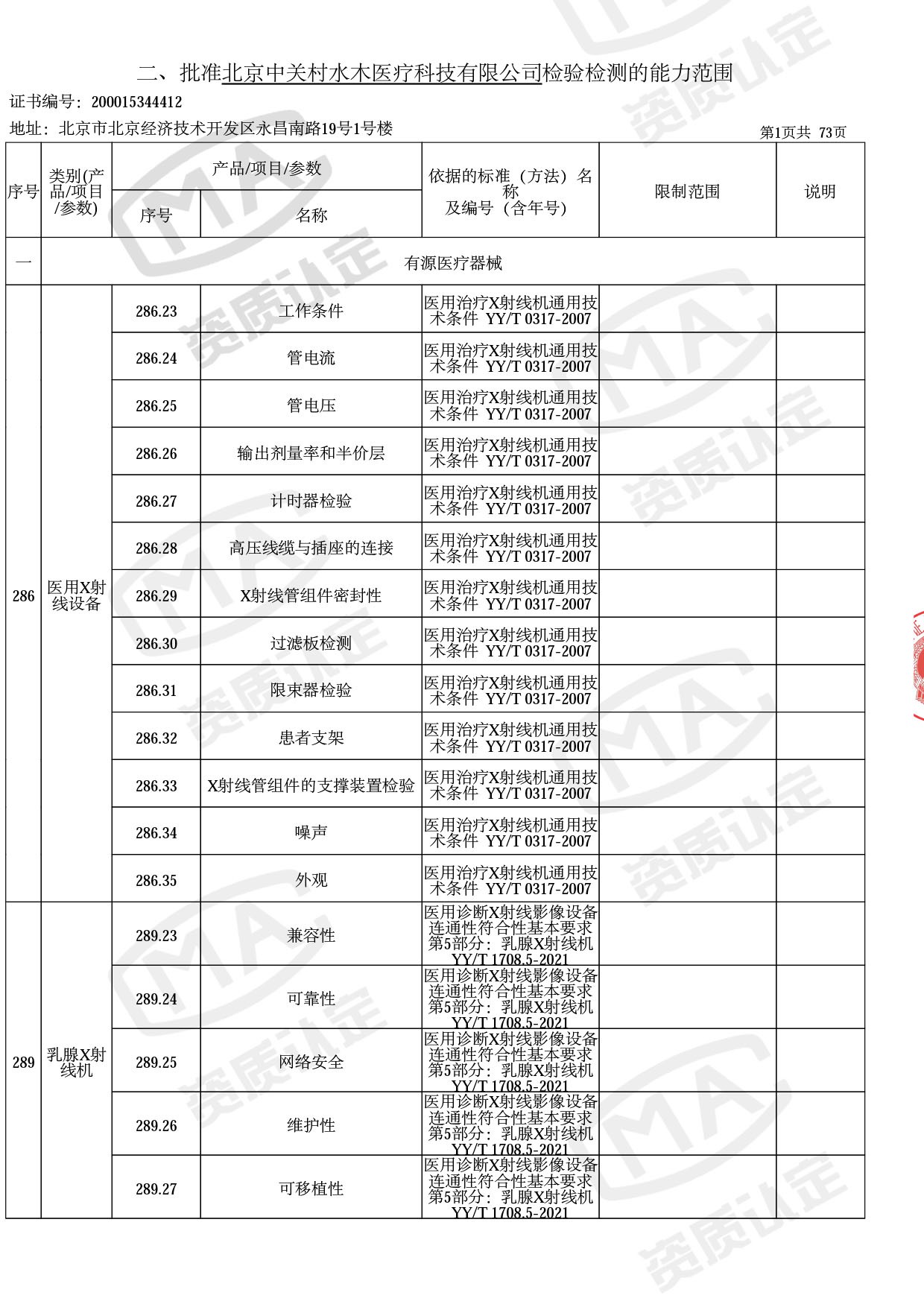
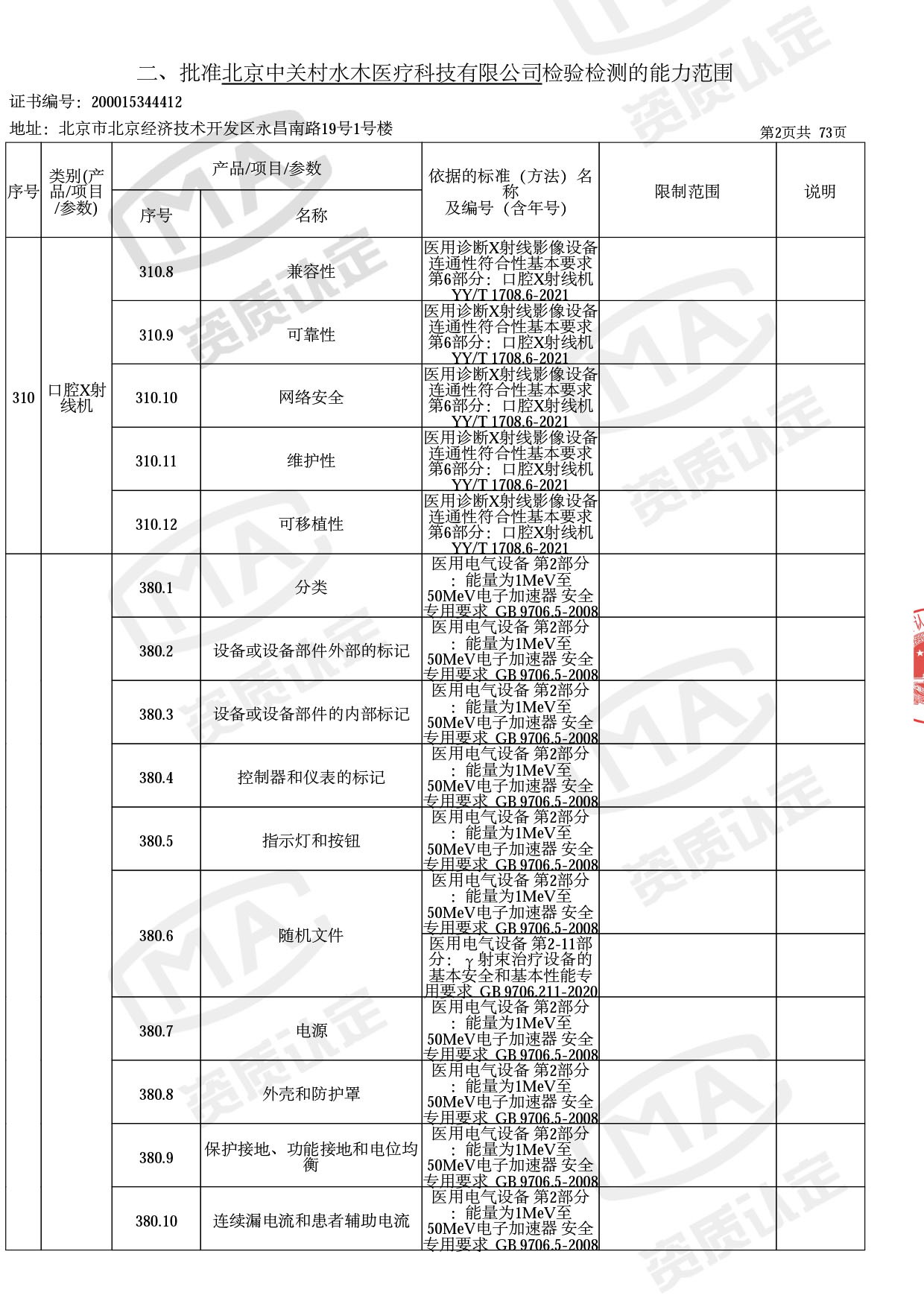
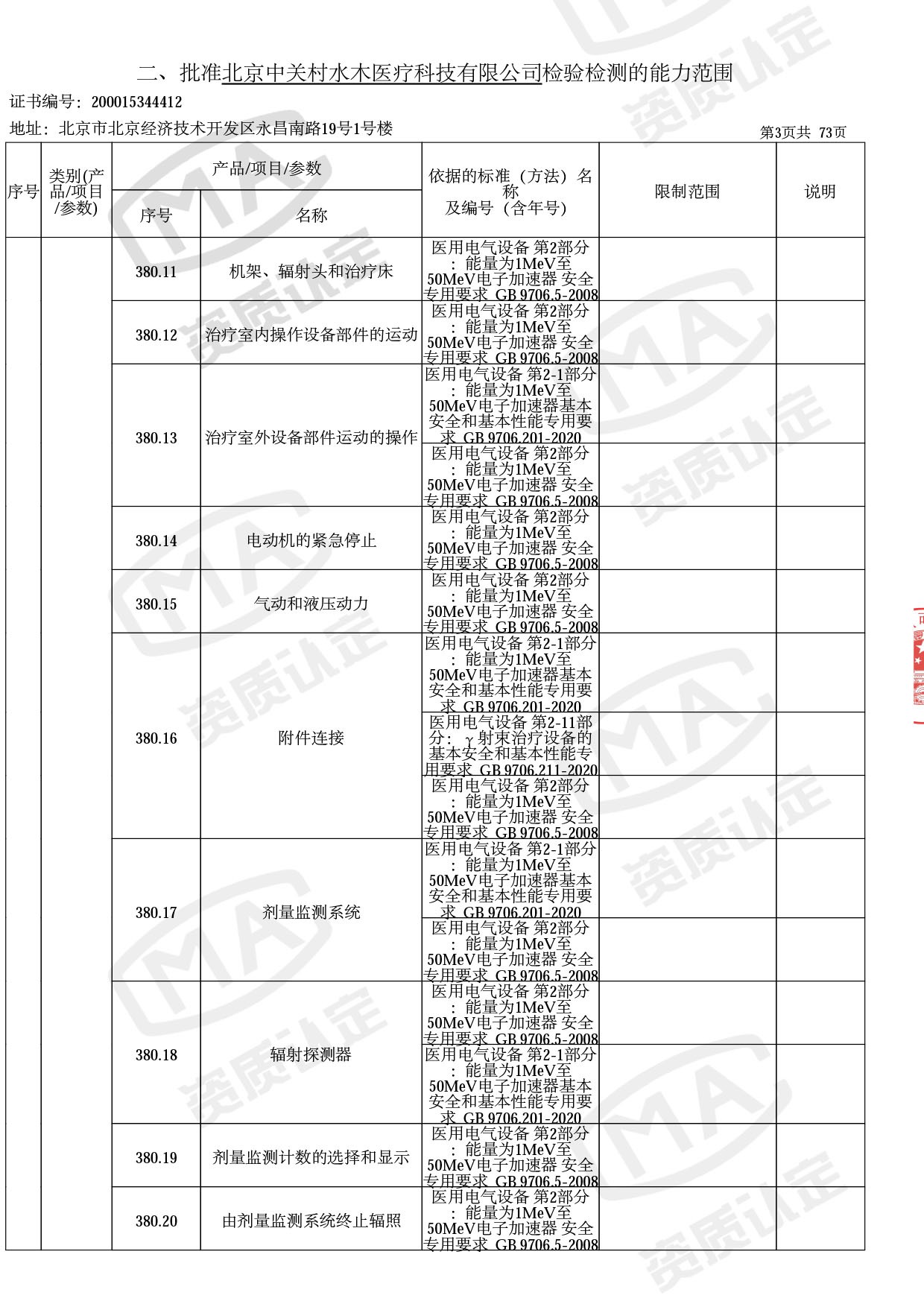
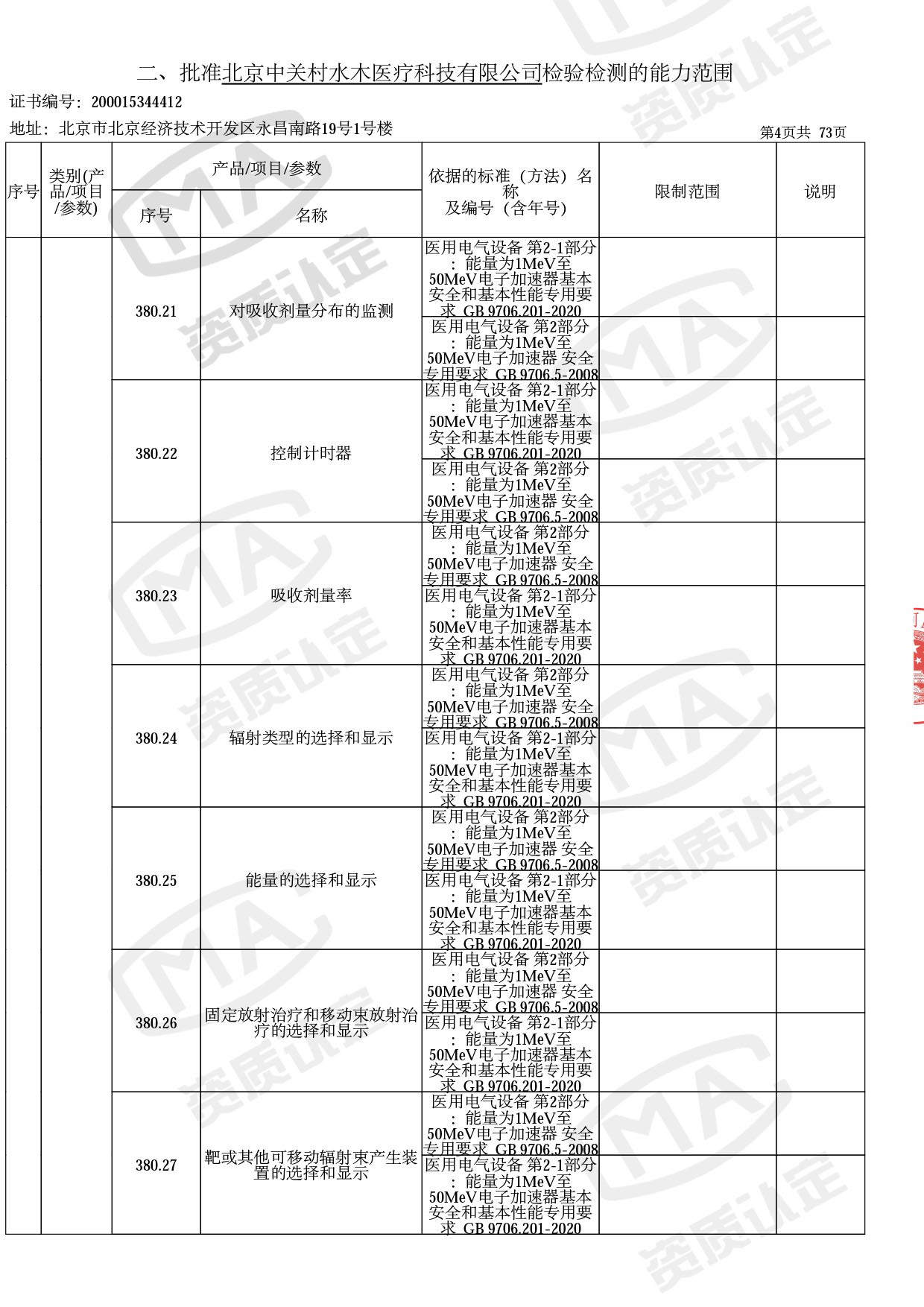
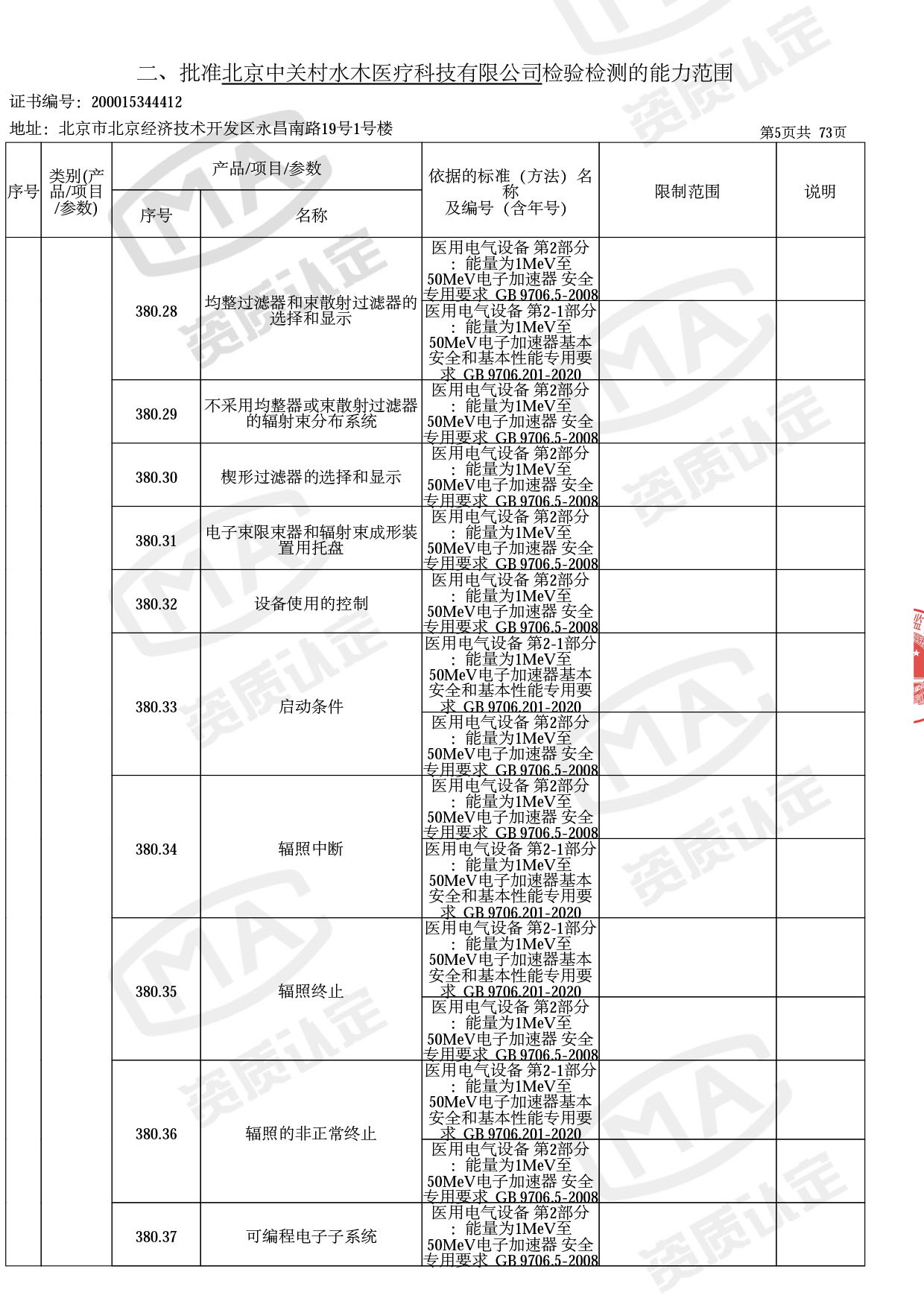
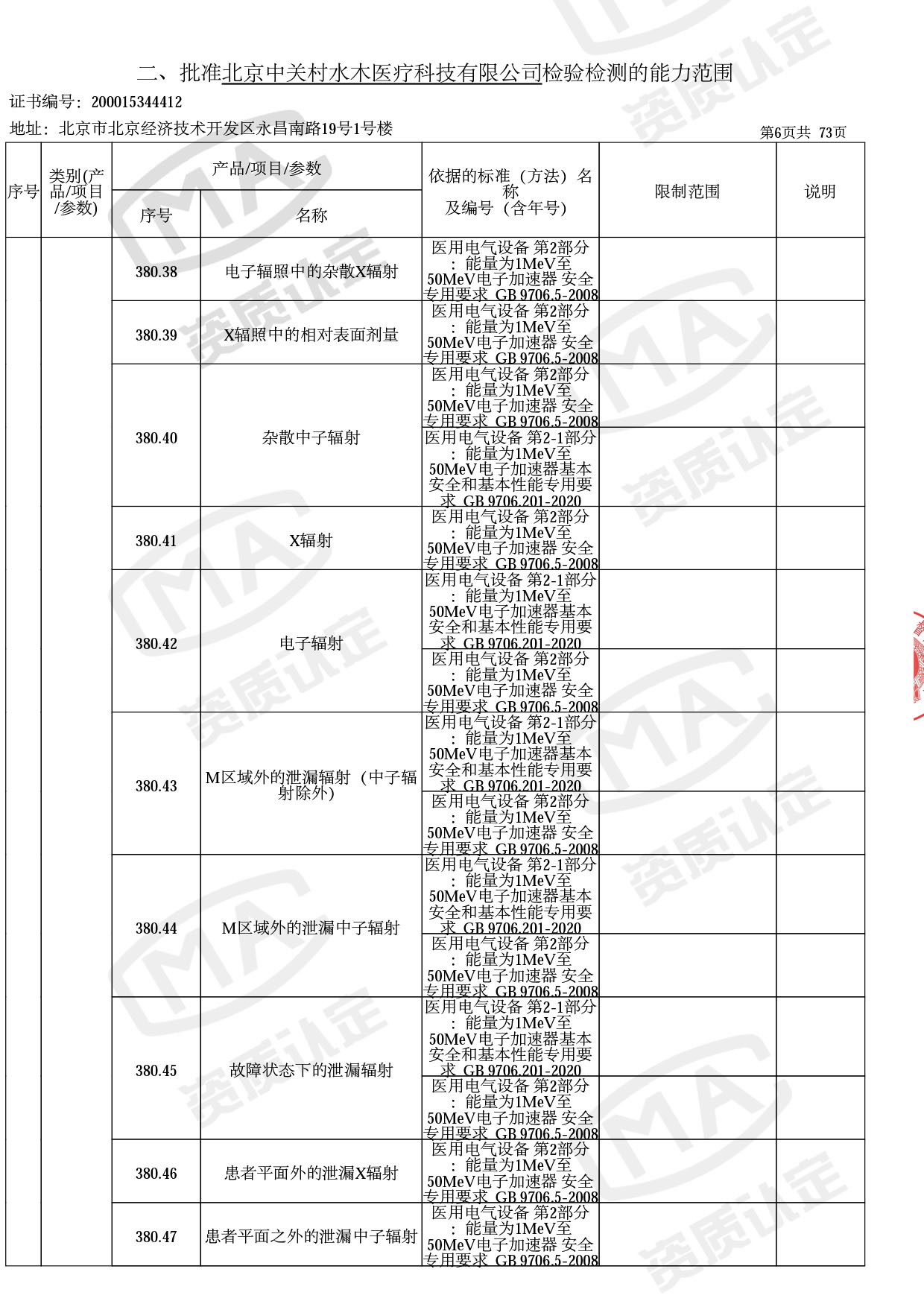
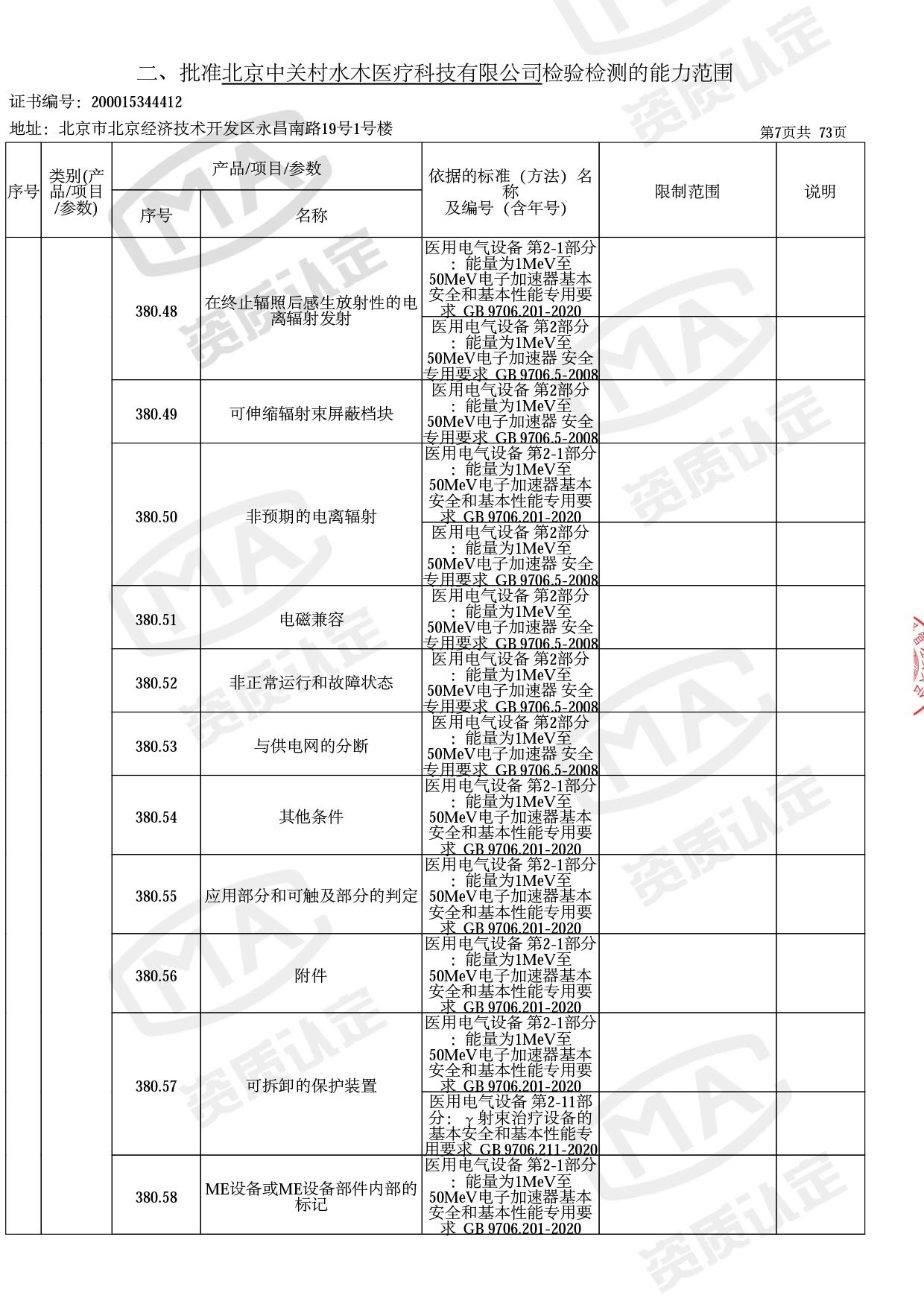
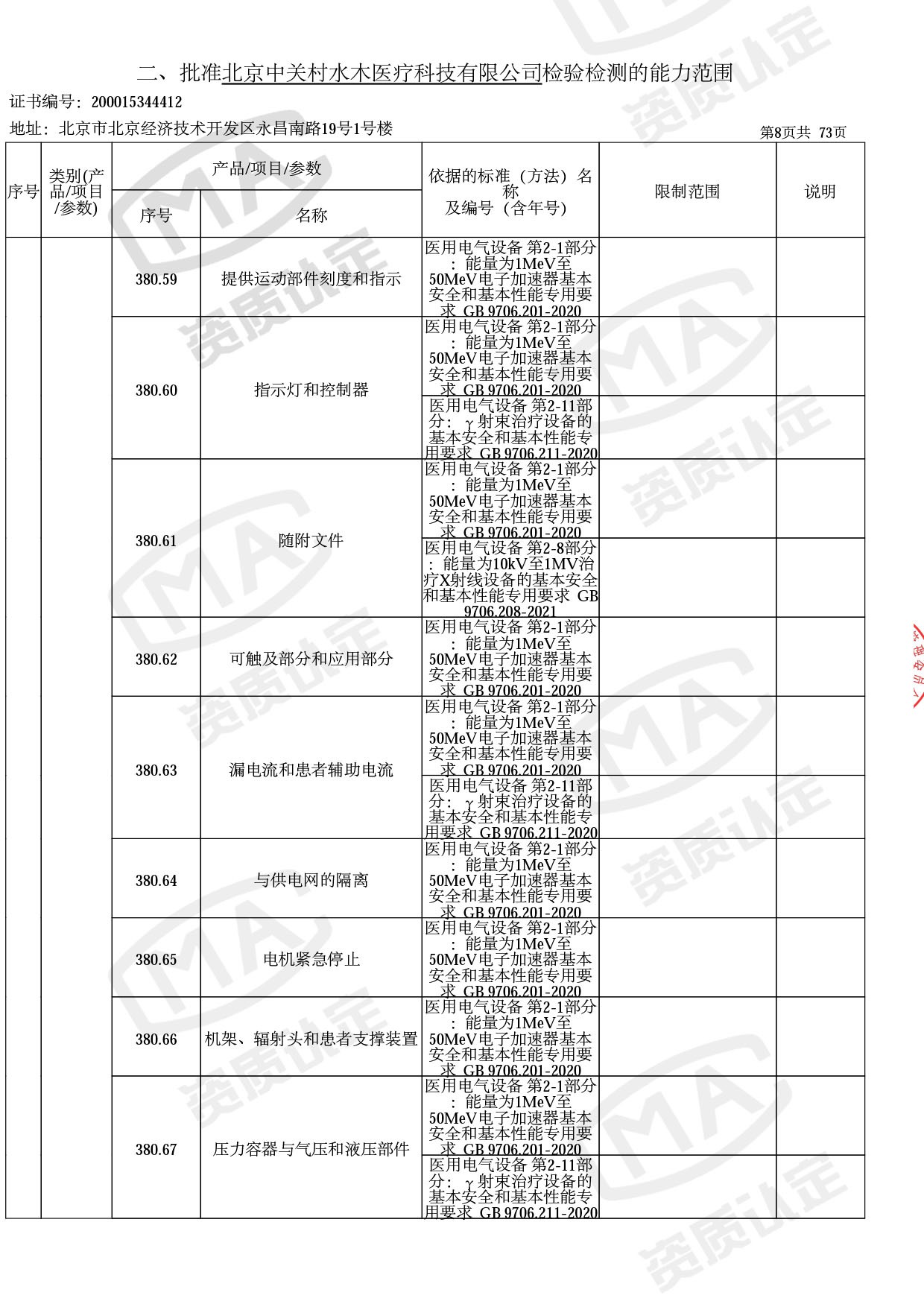
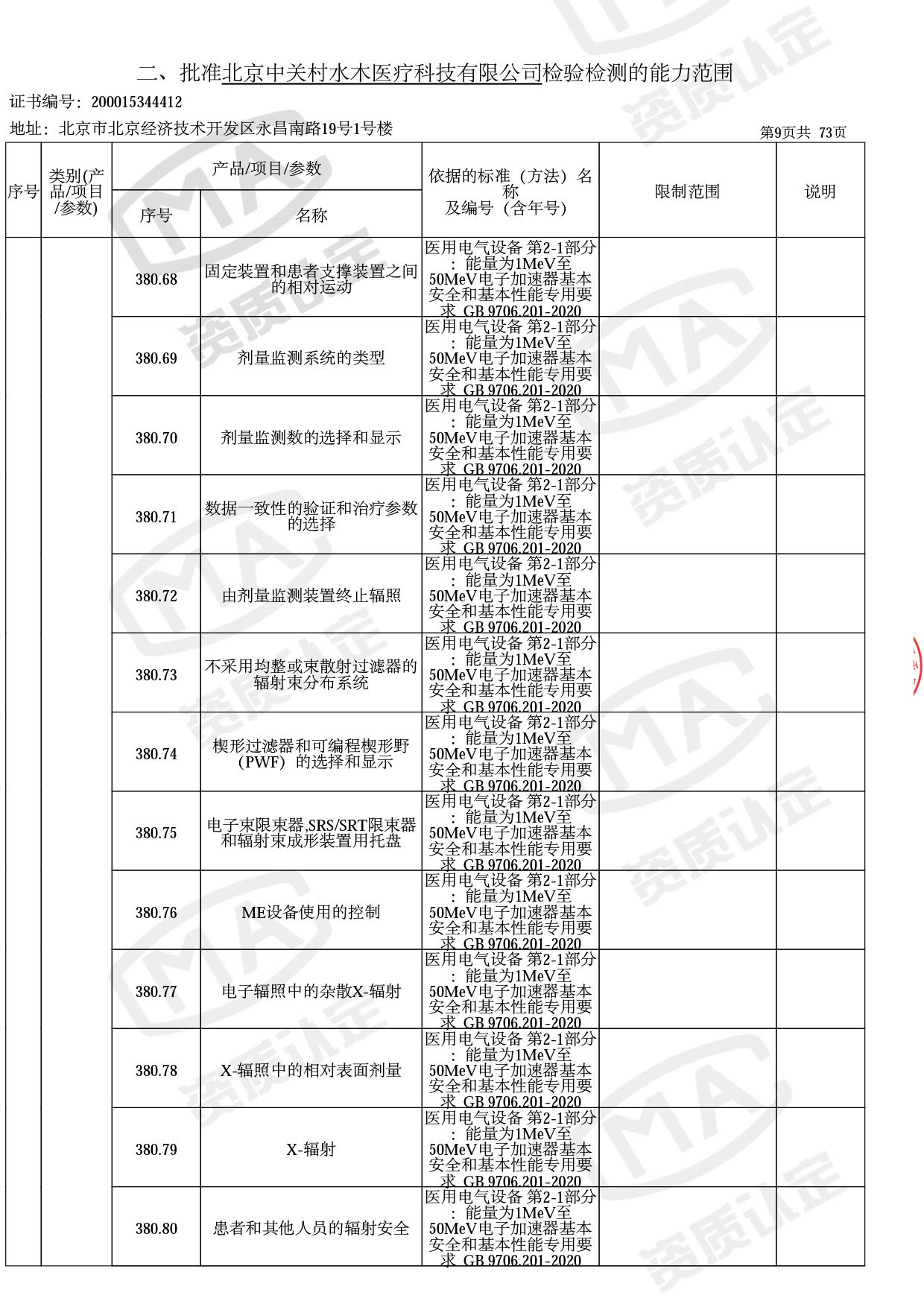
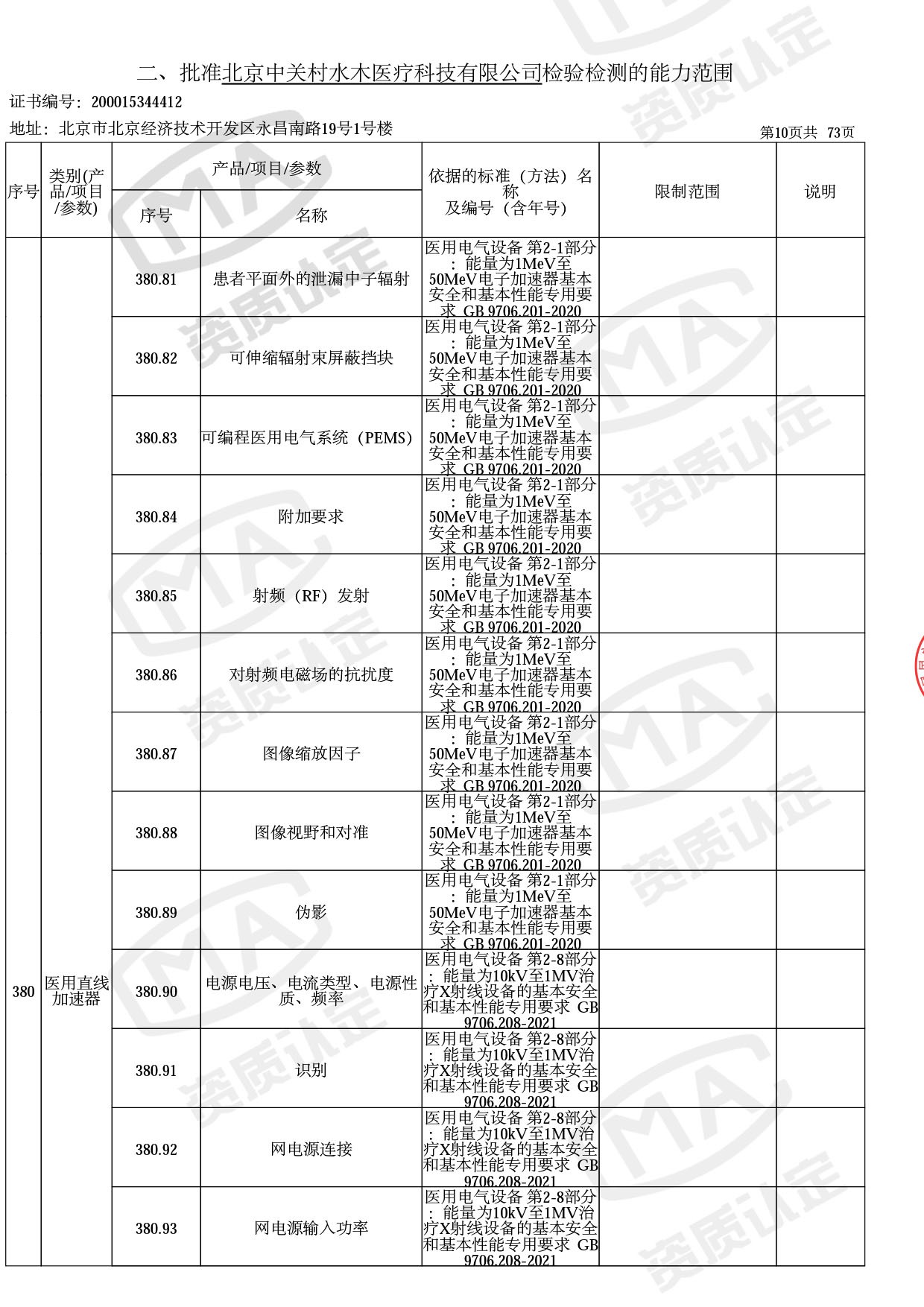
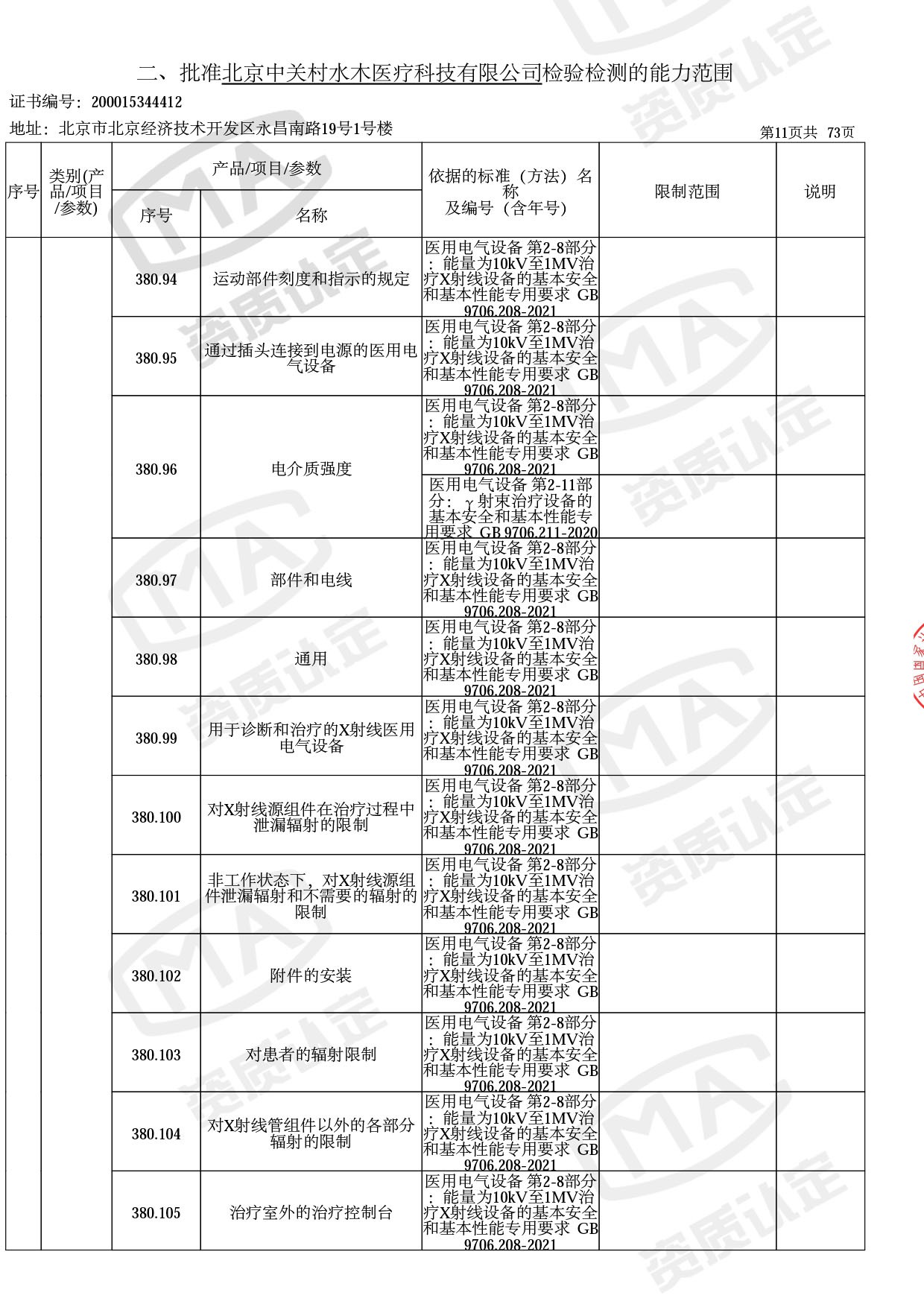
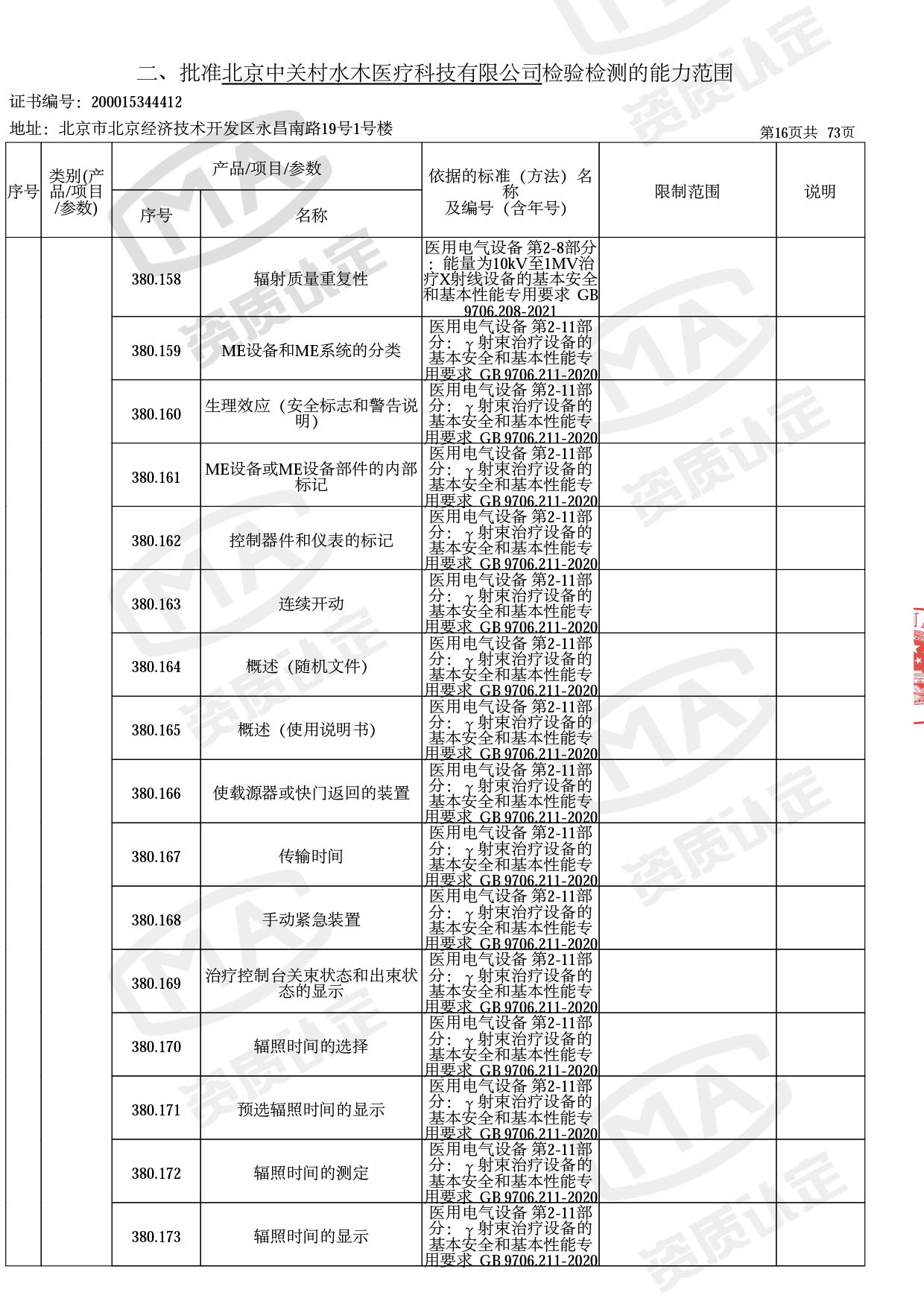
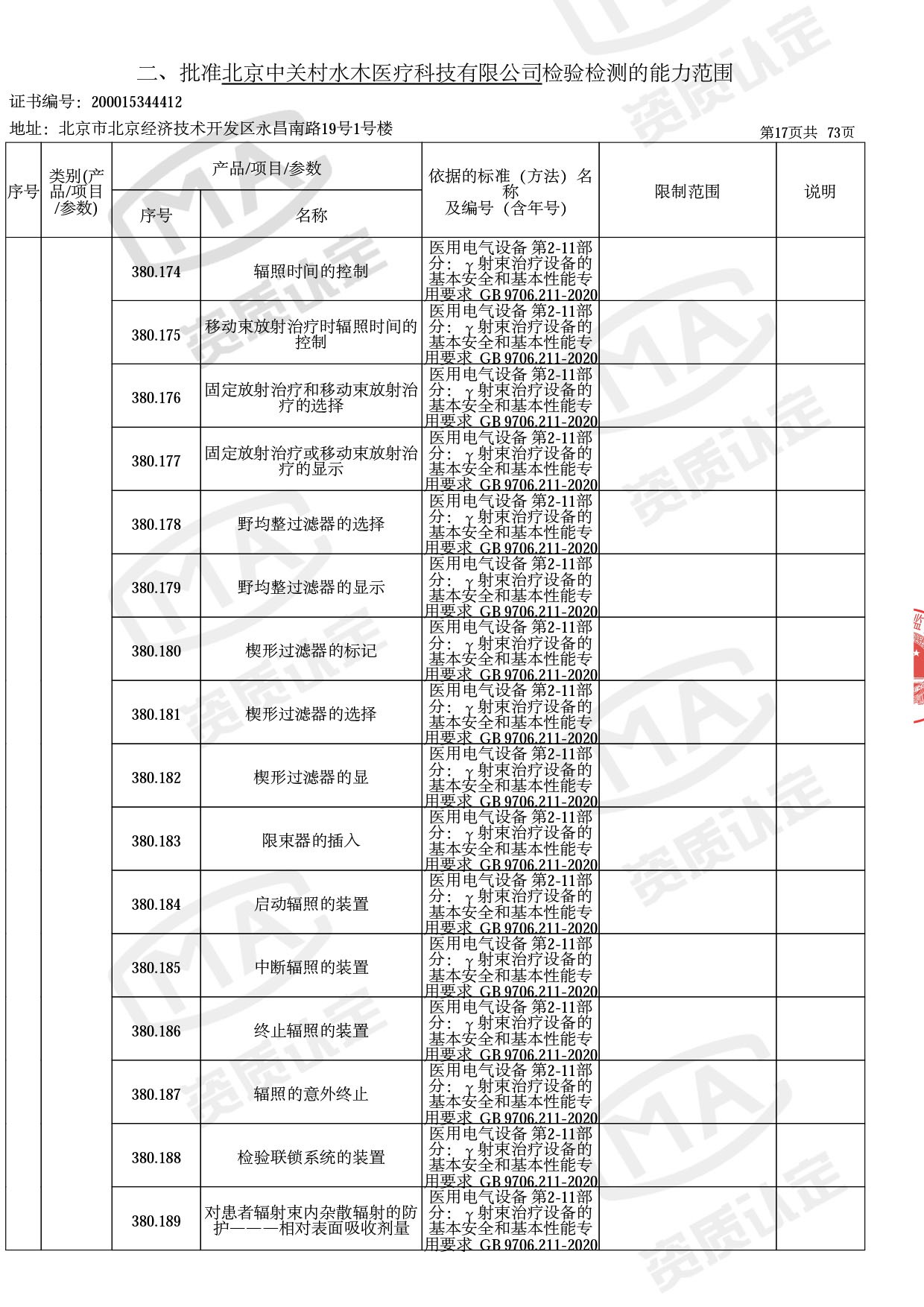
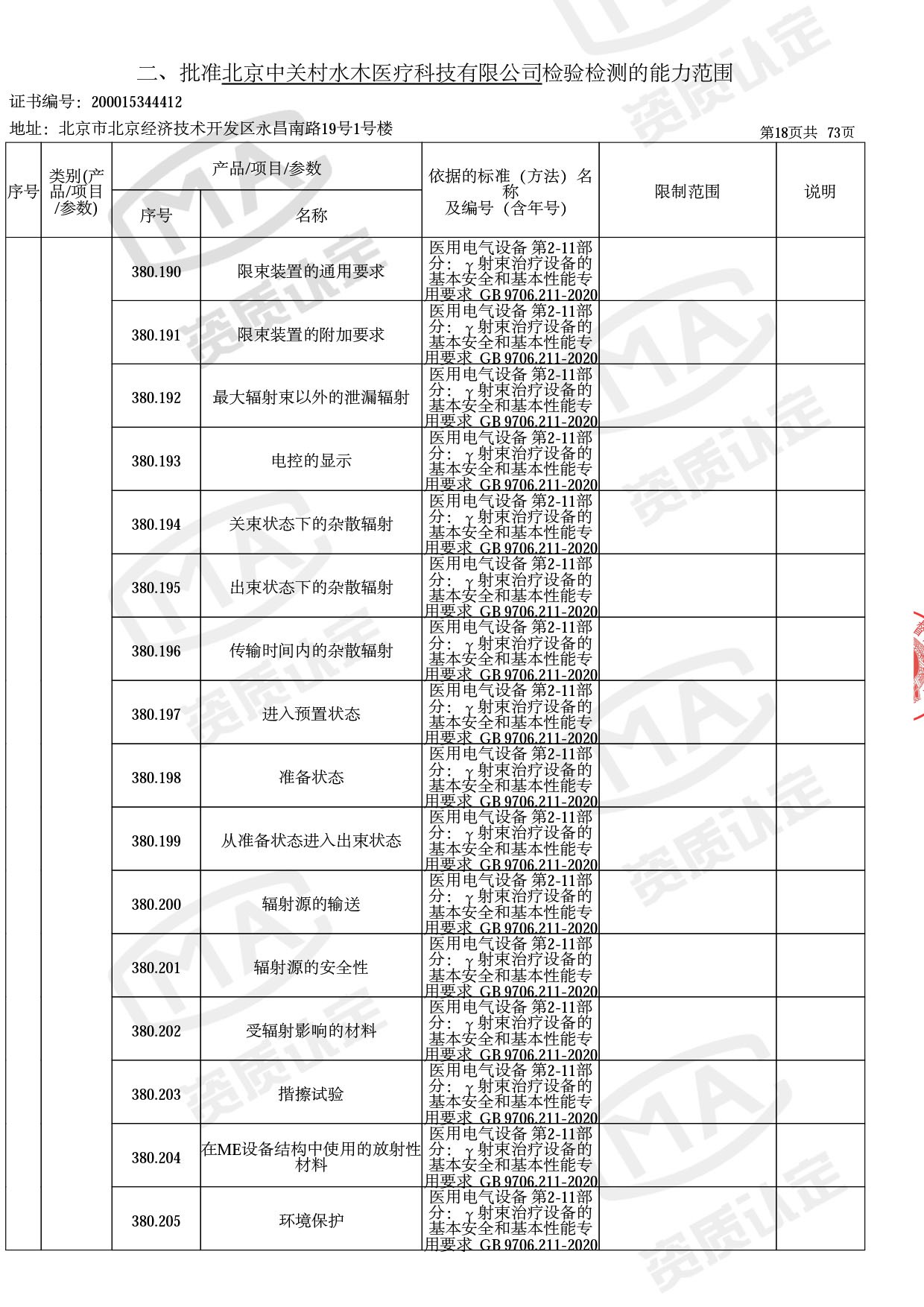
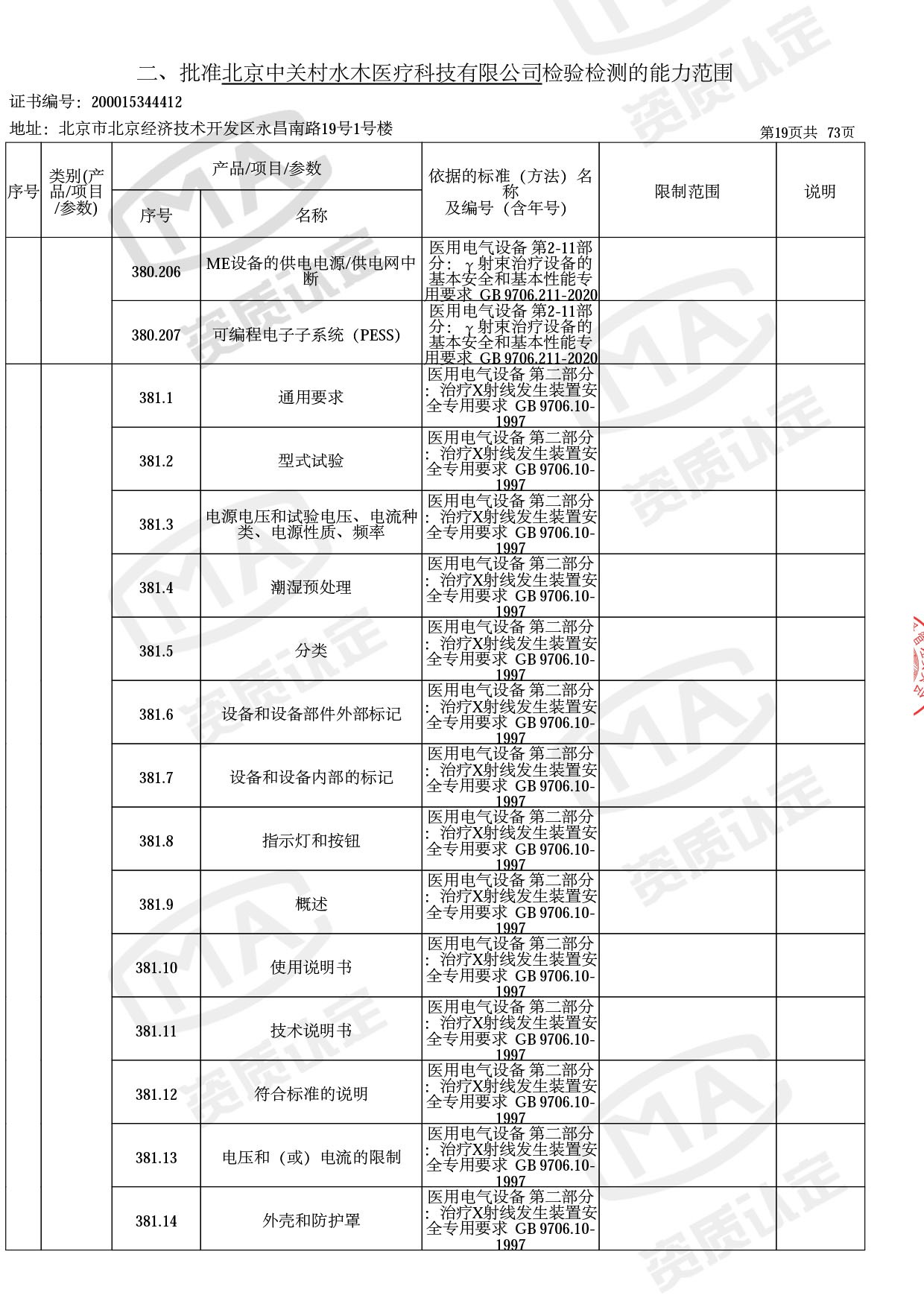
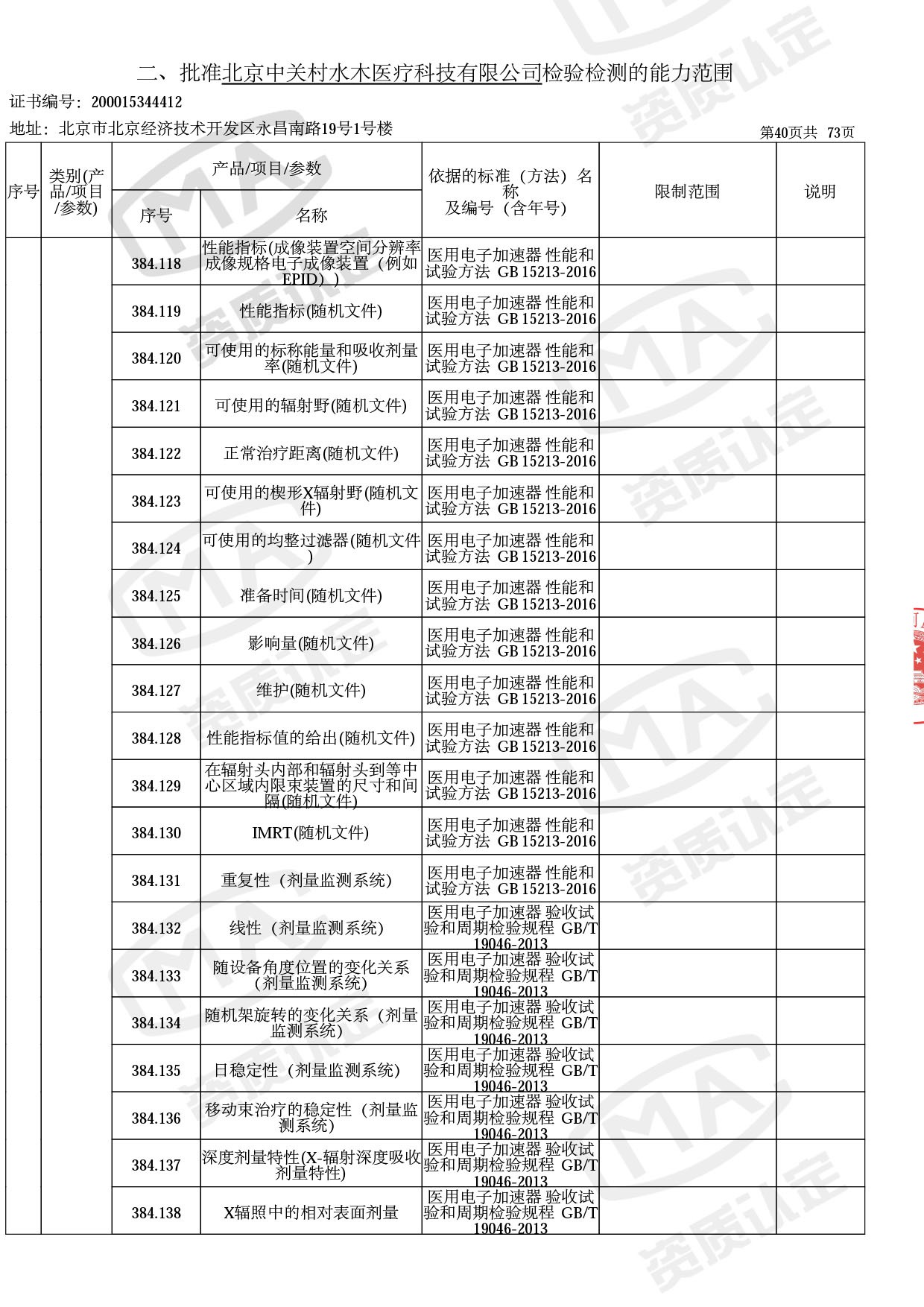
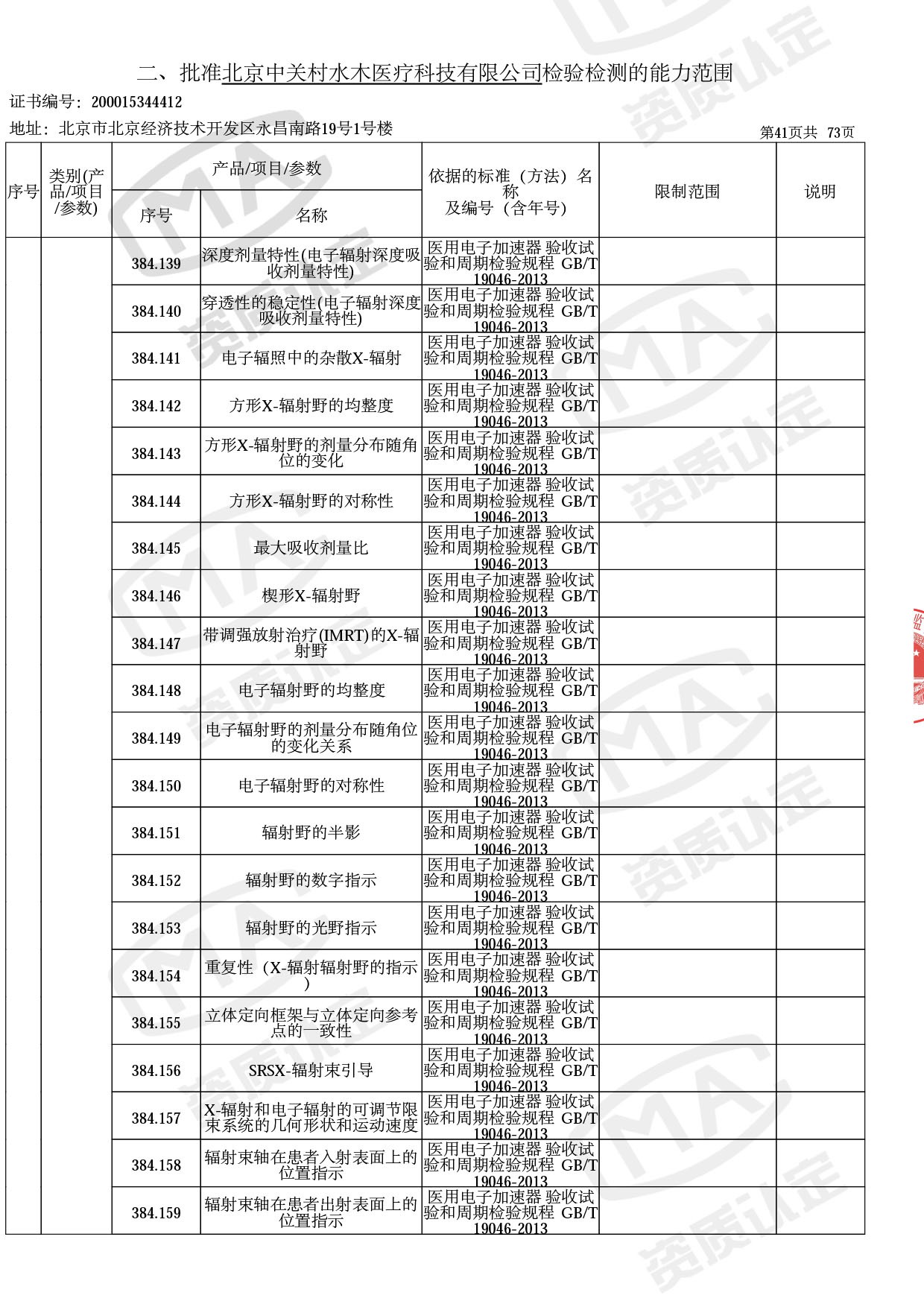
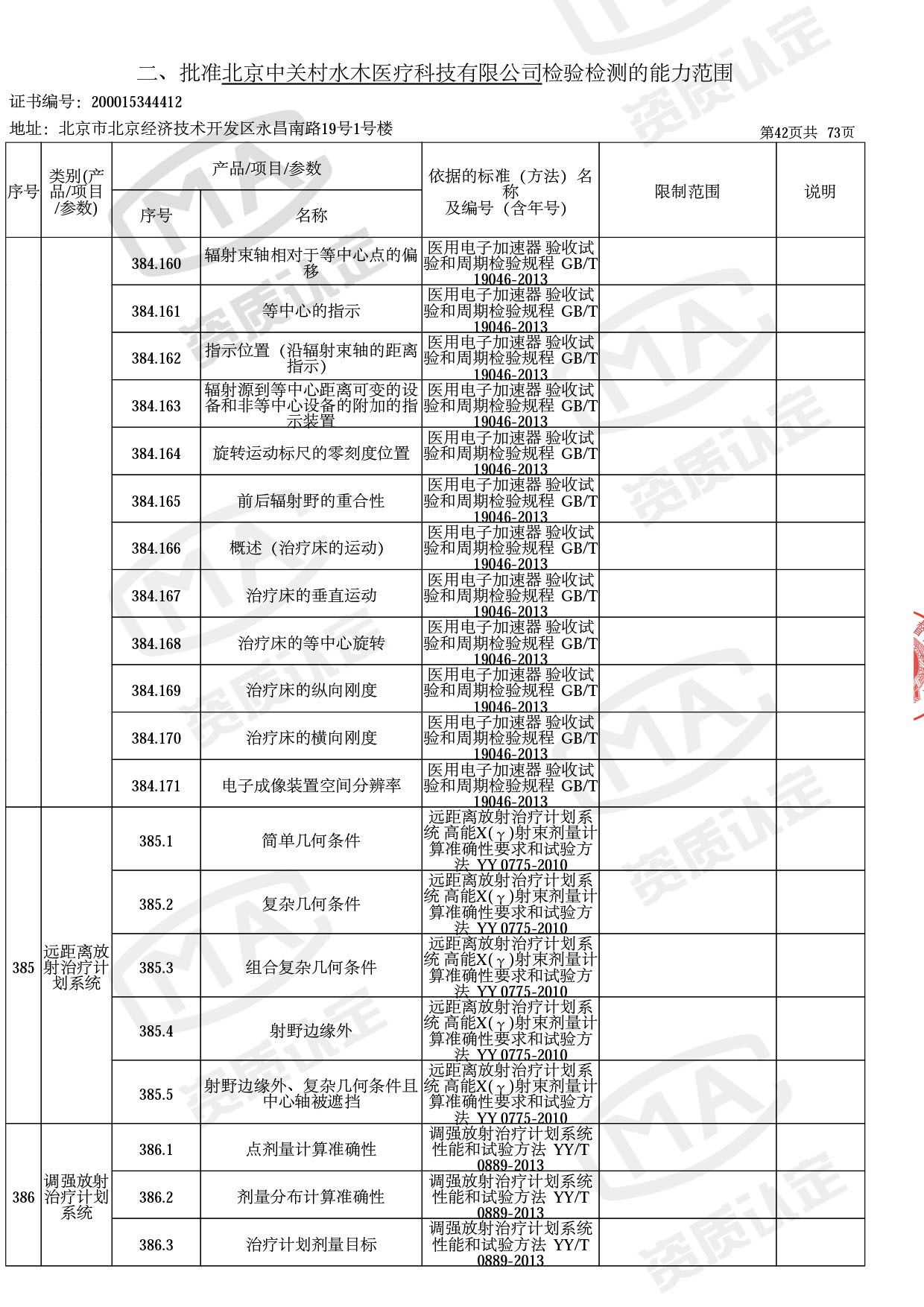
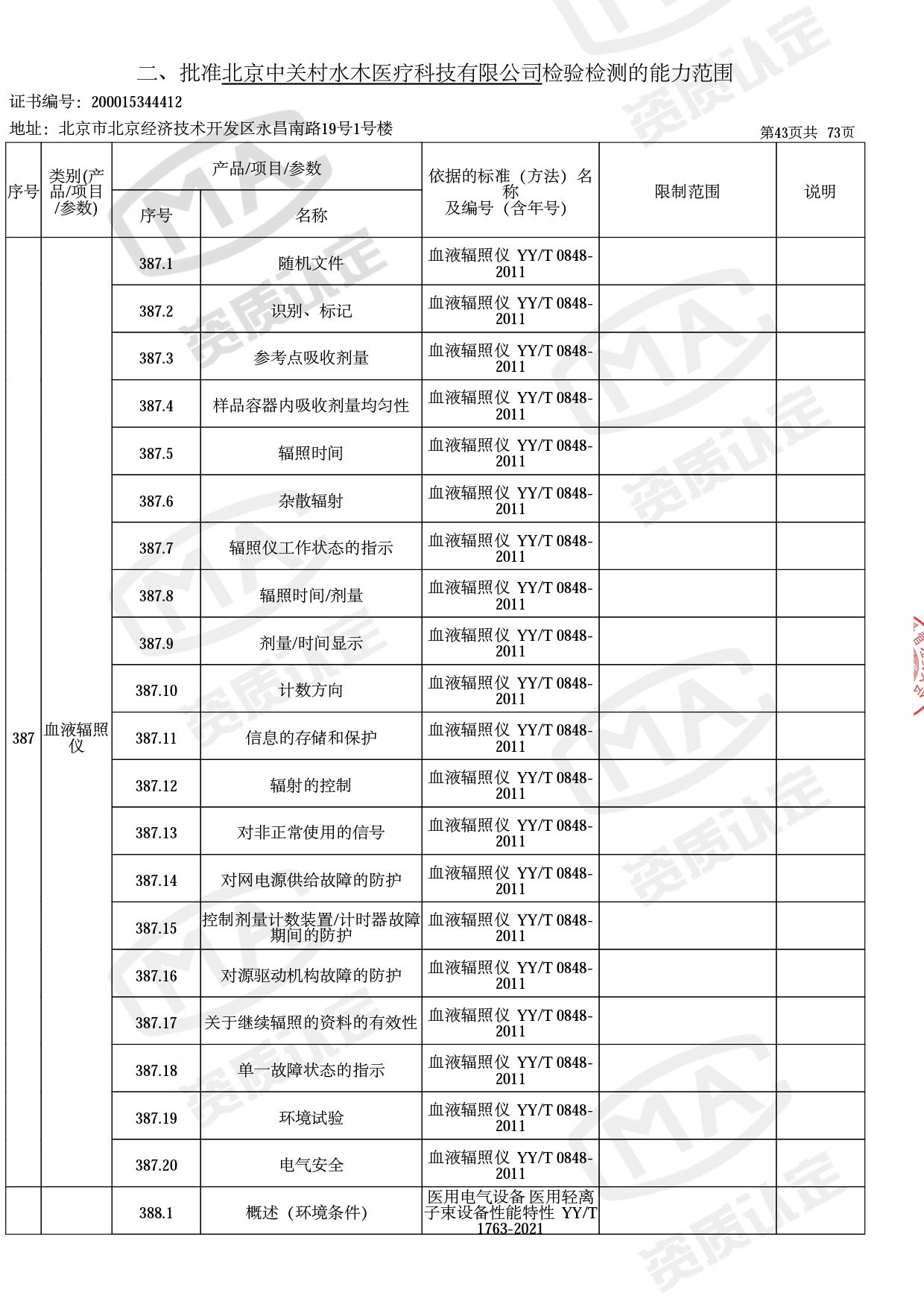
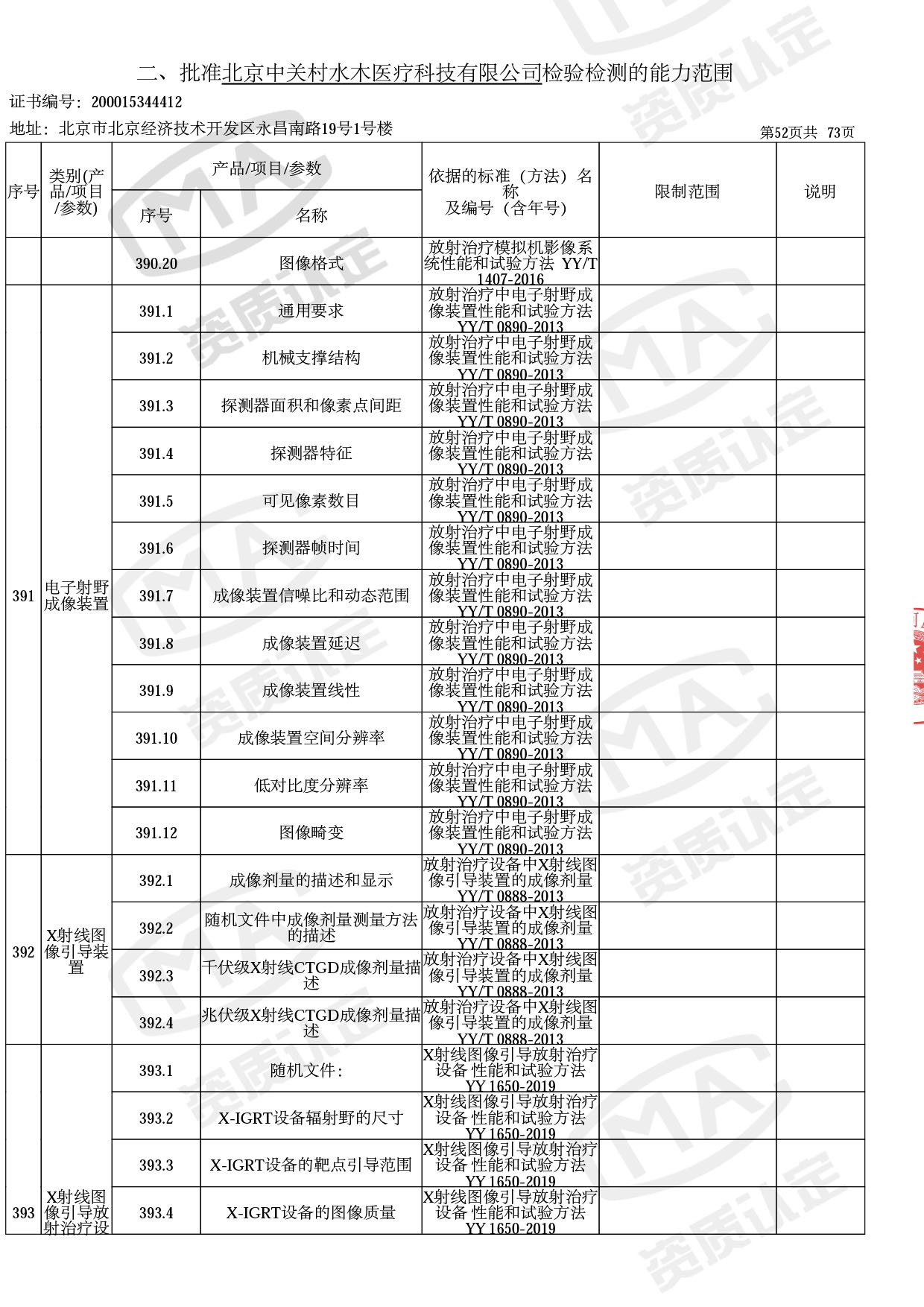
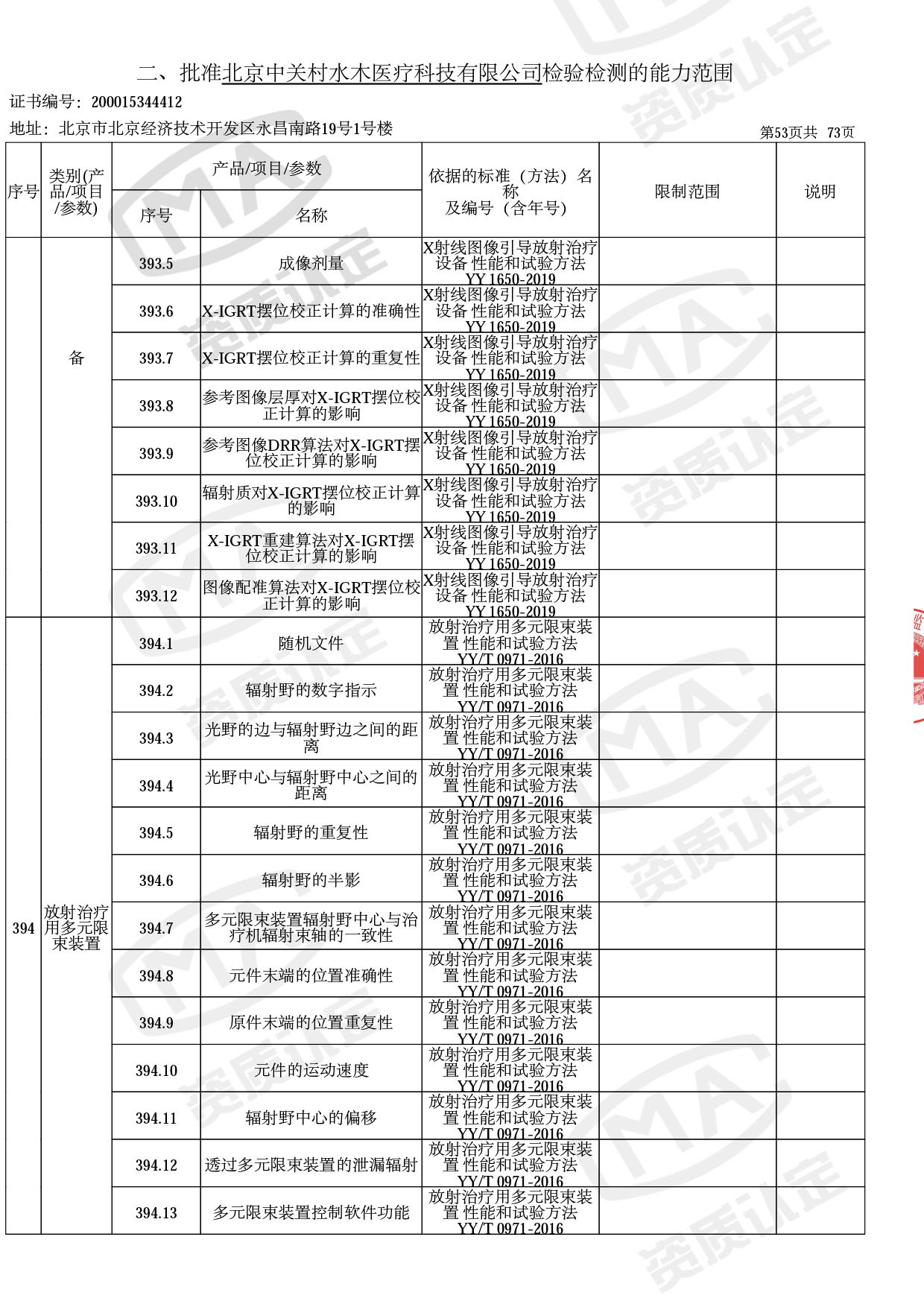
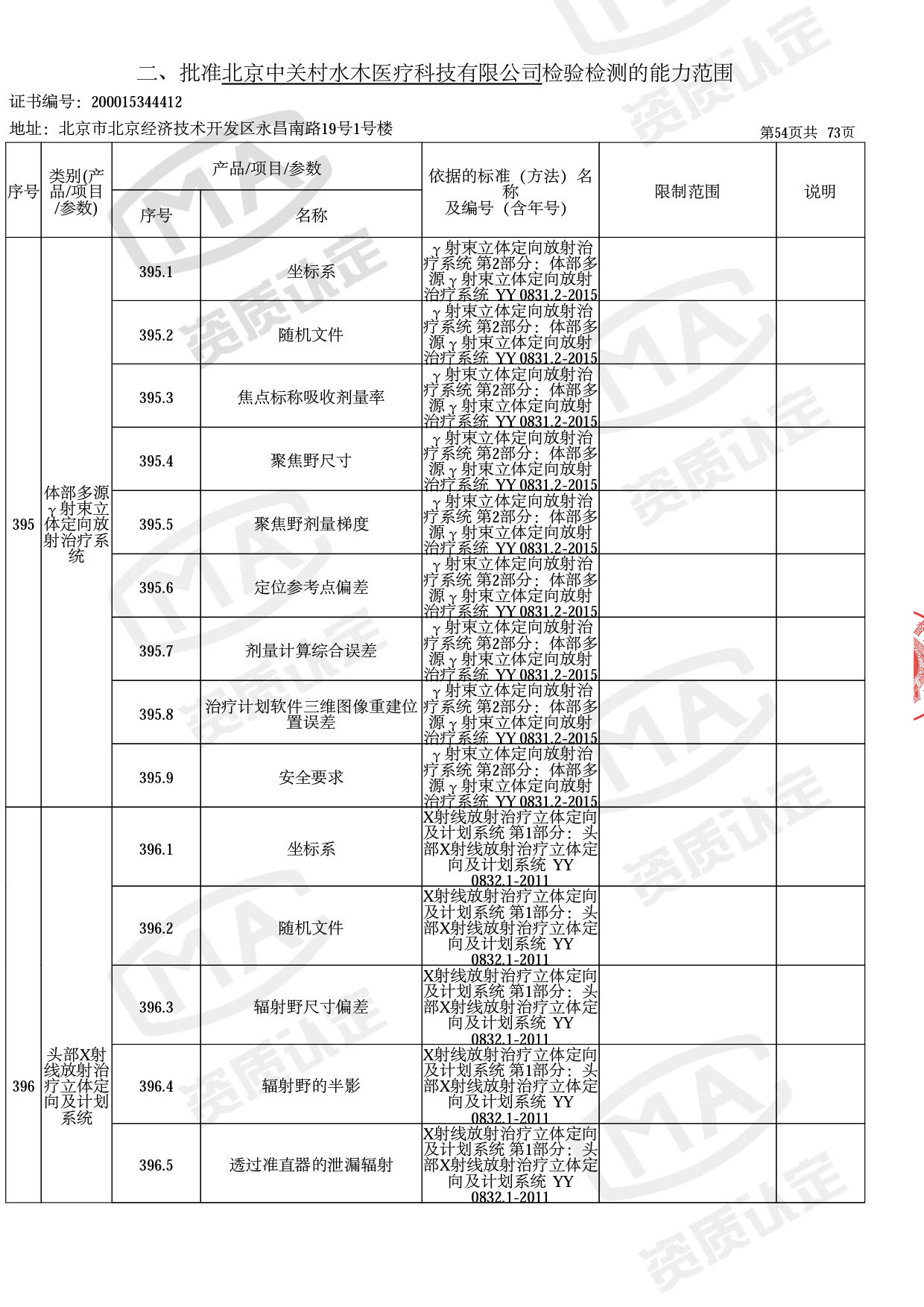
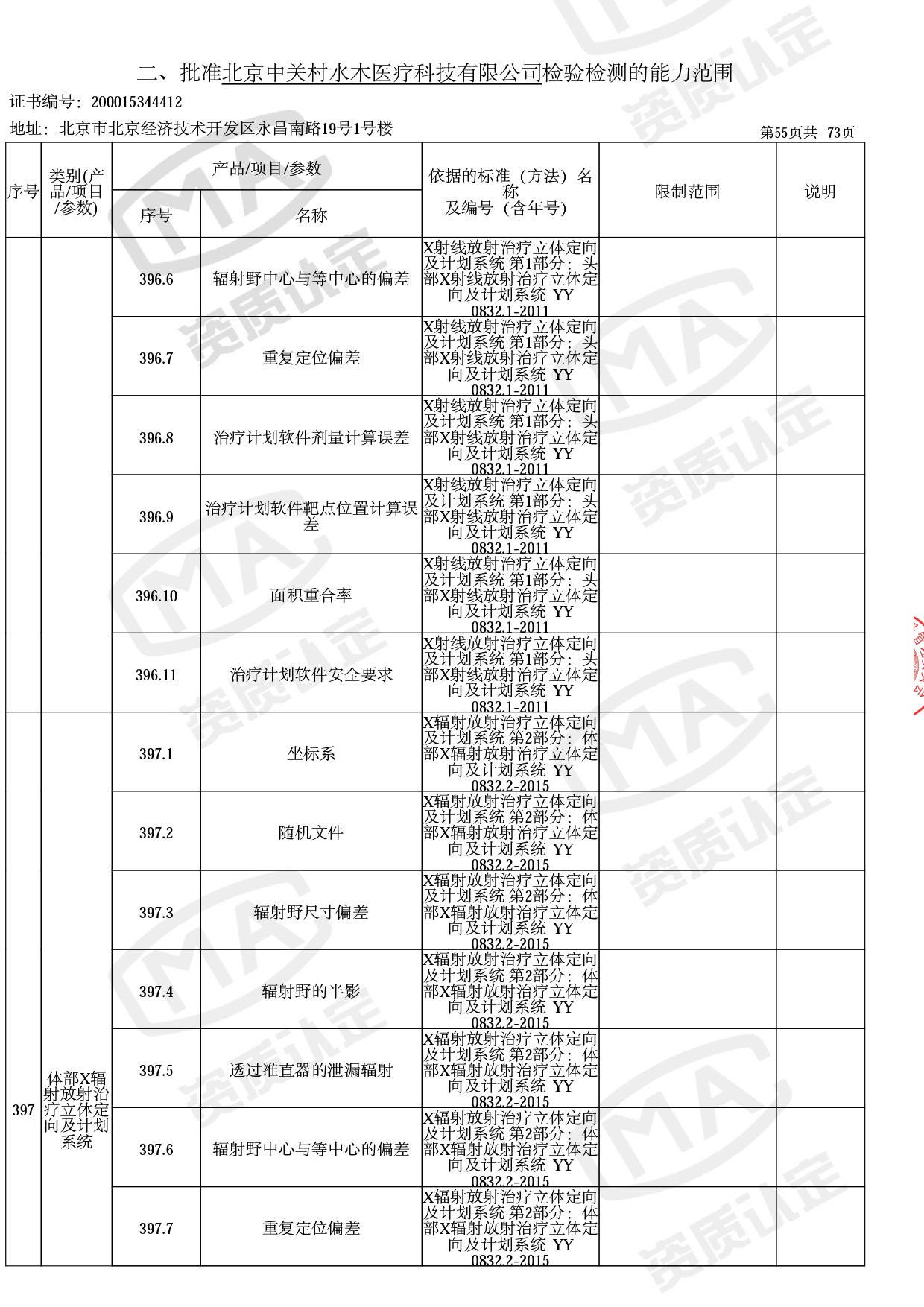
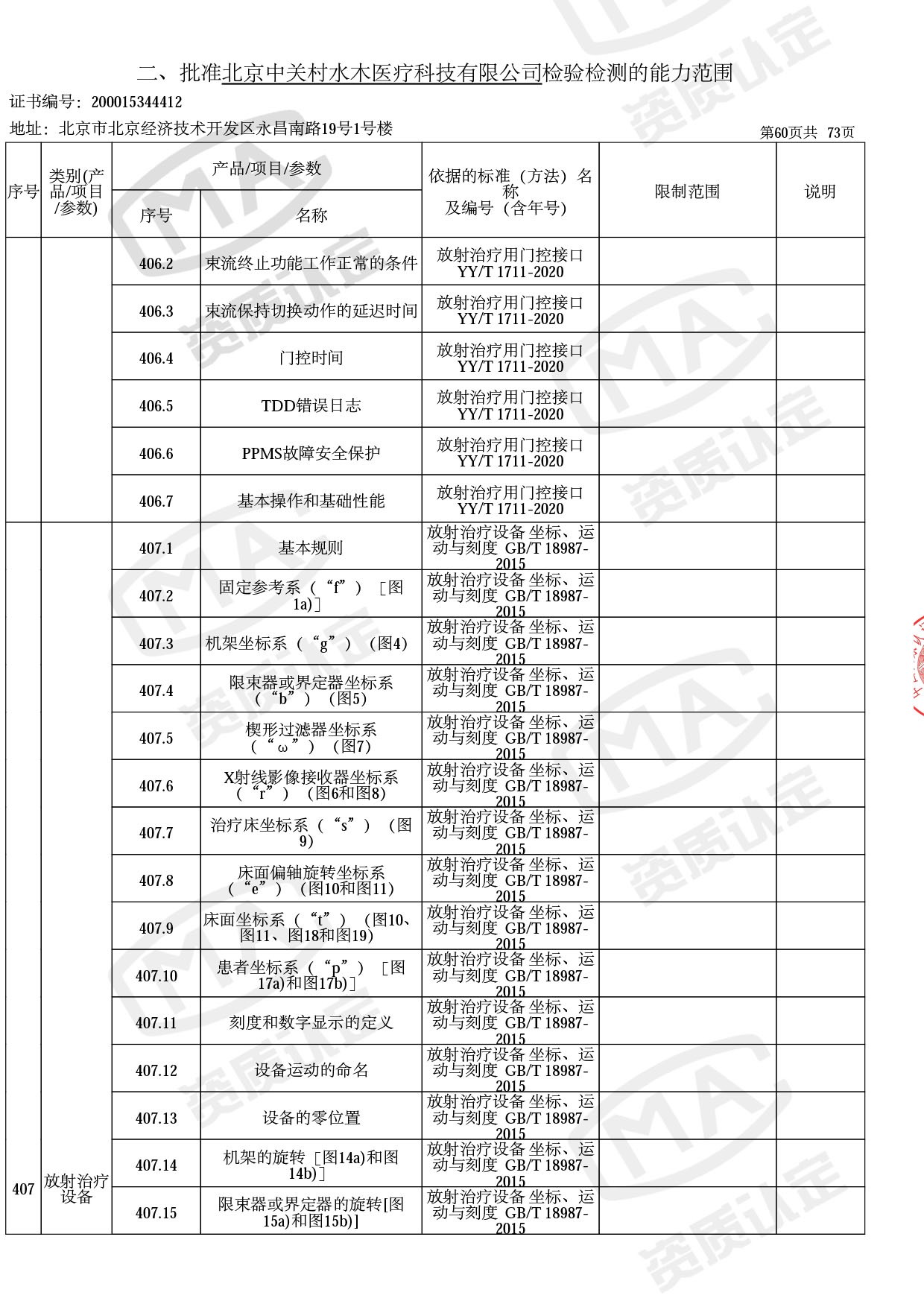
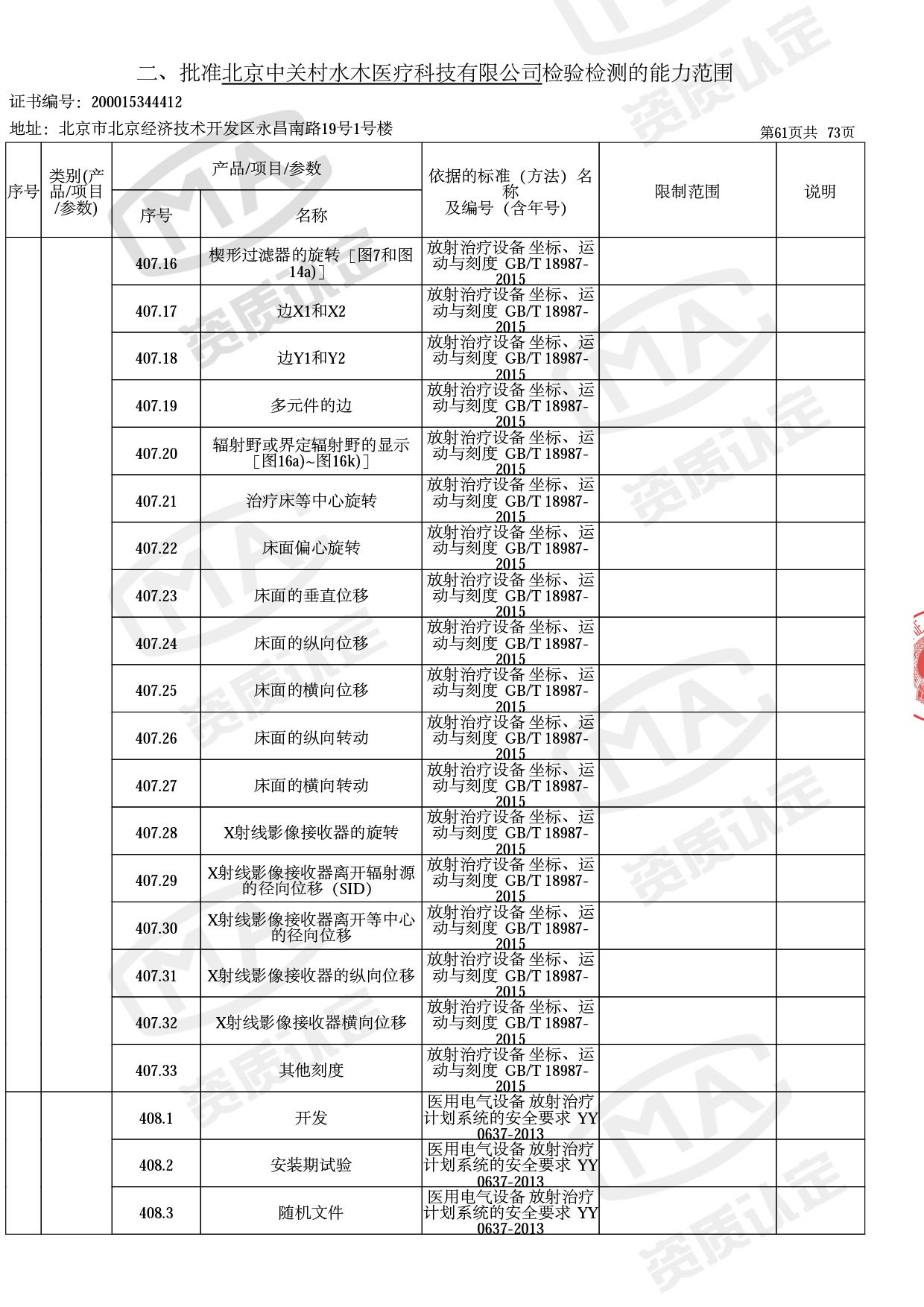
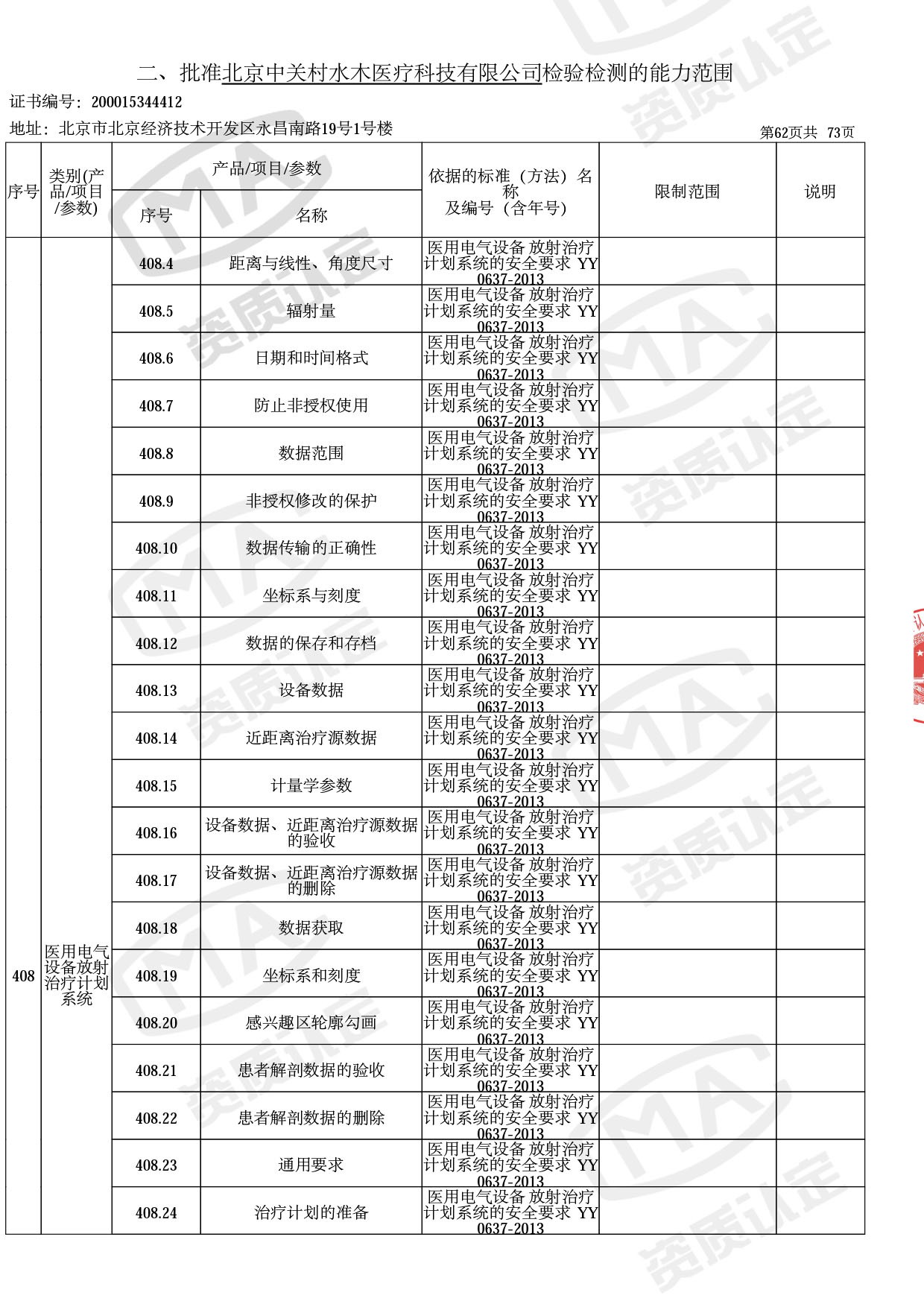
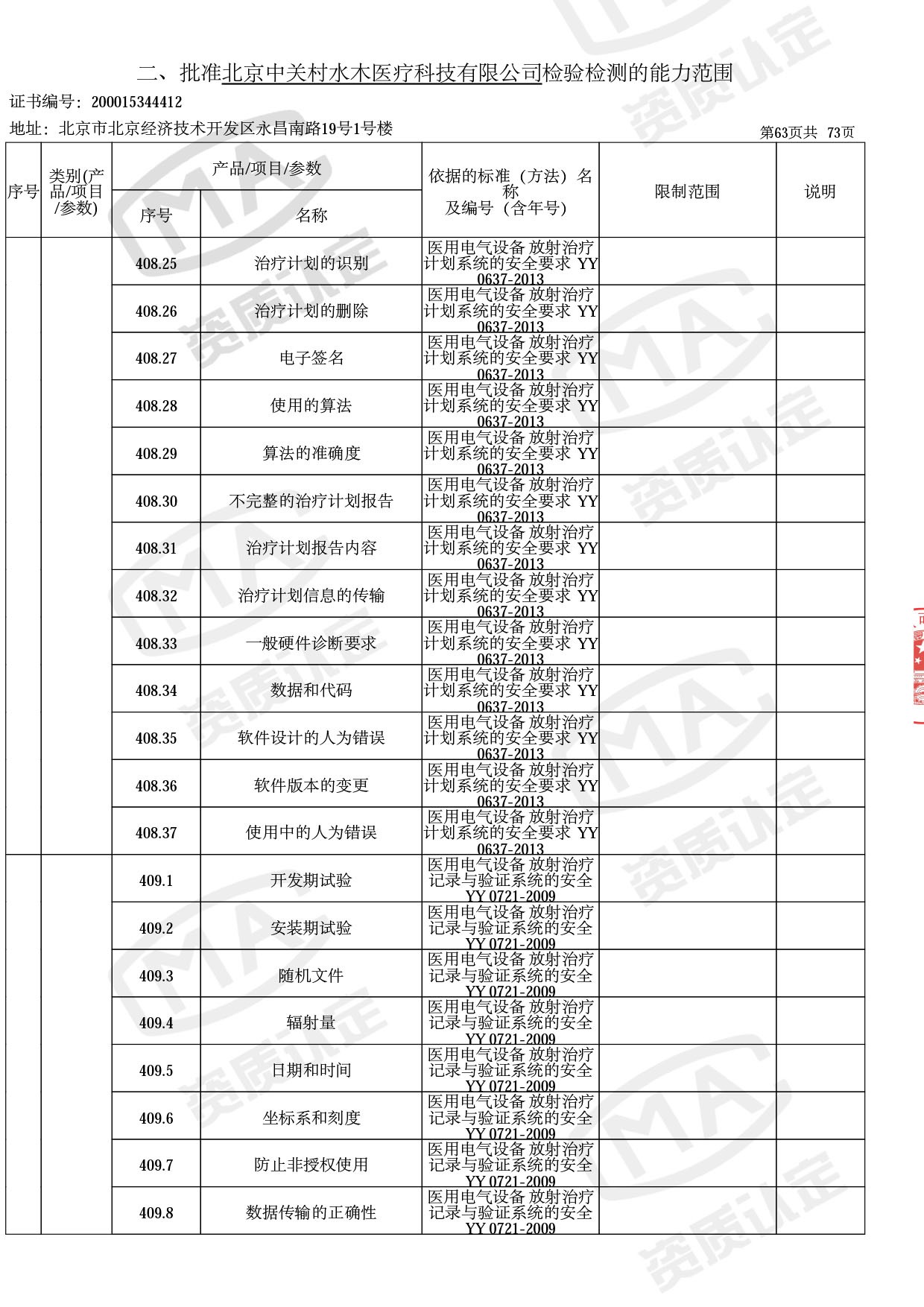
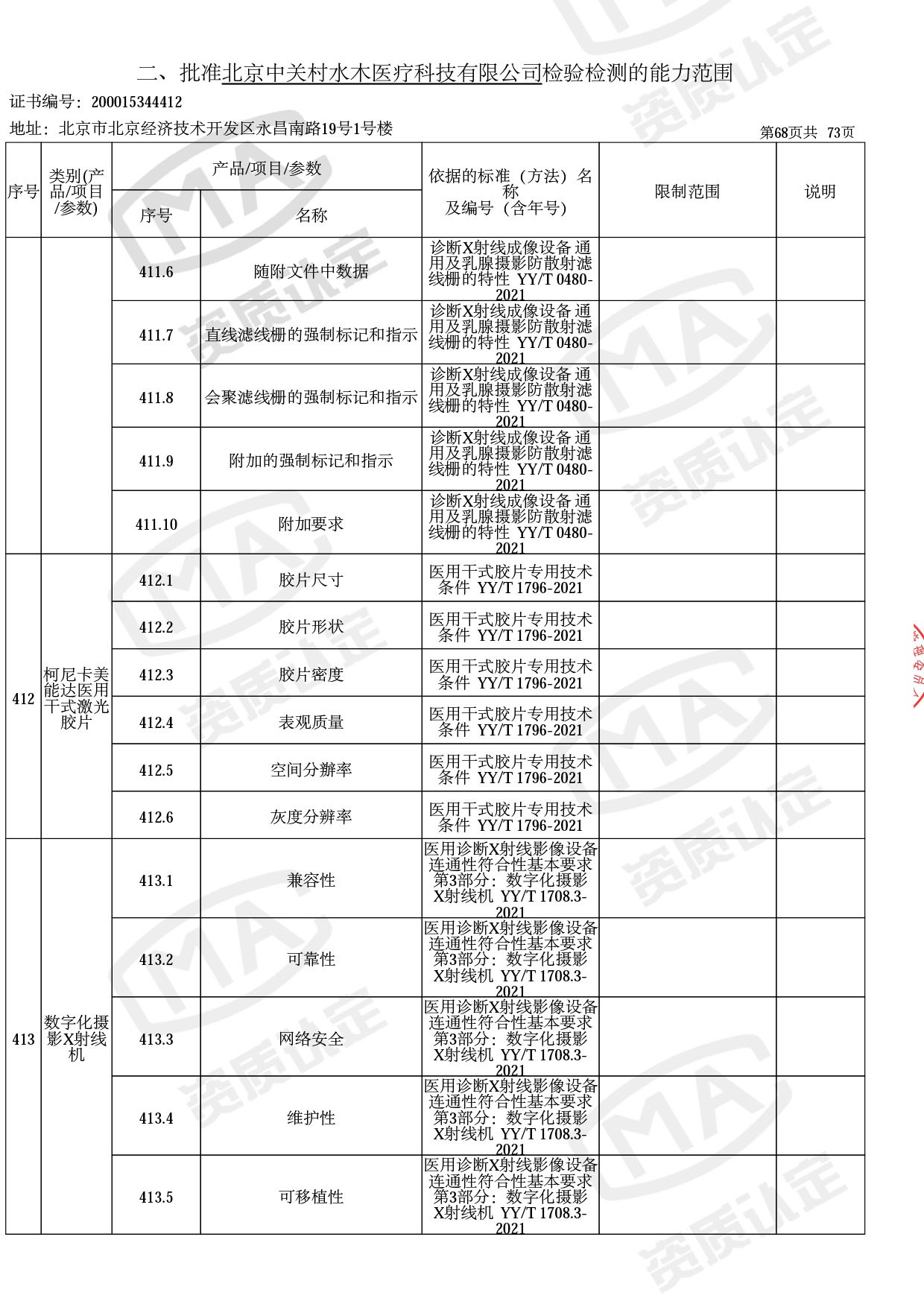
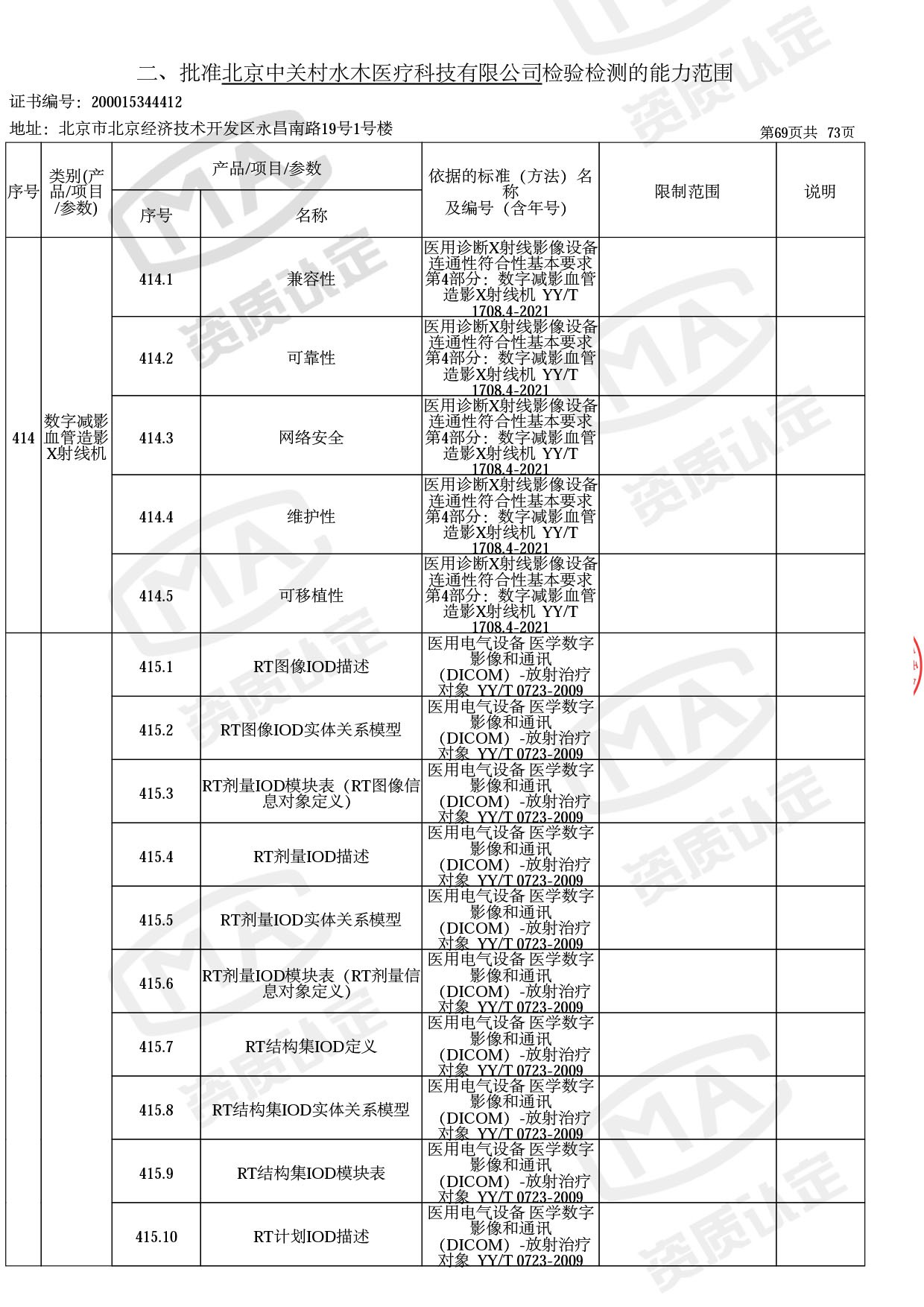
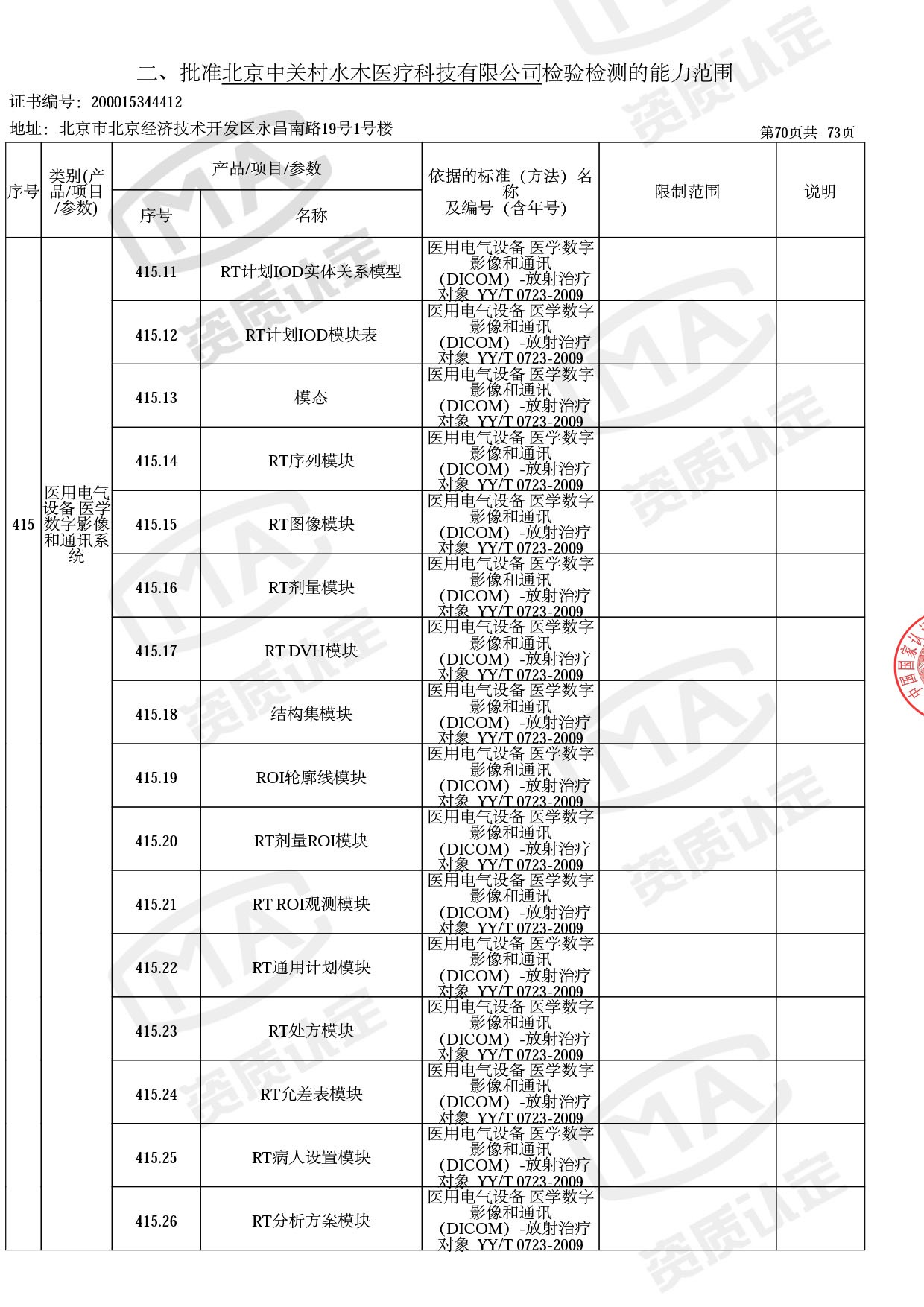
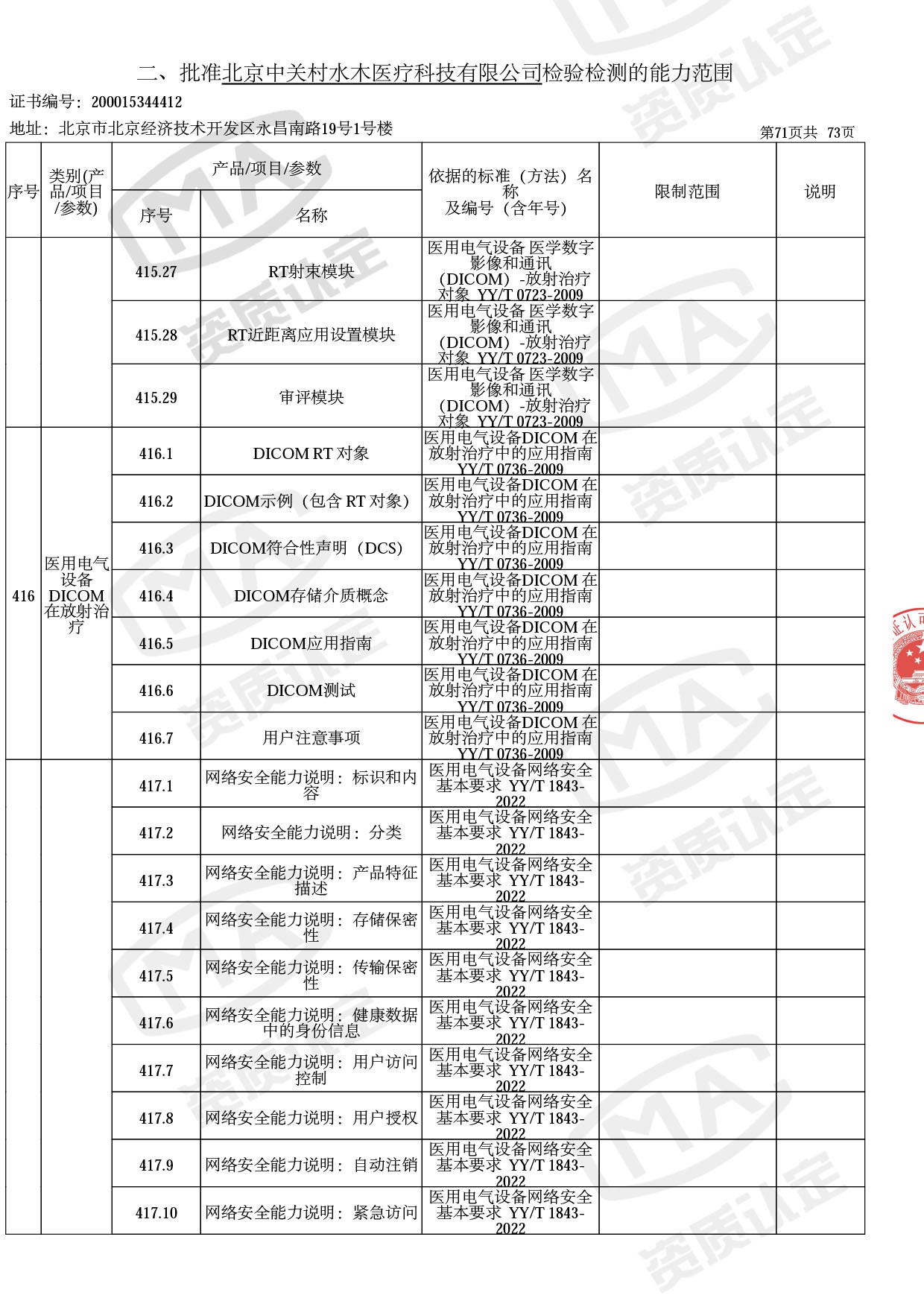
◎ 本次扩项涉及新增产品类别 ◎
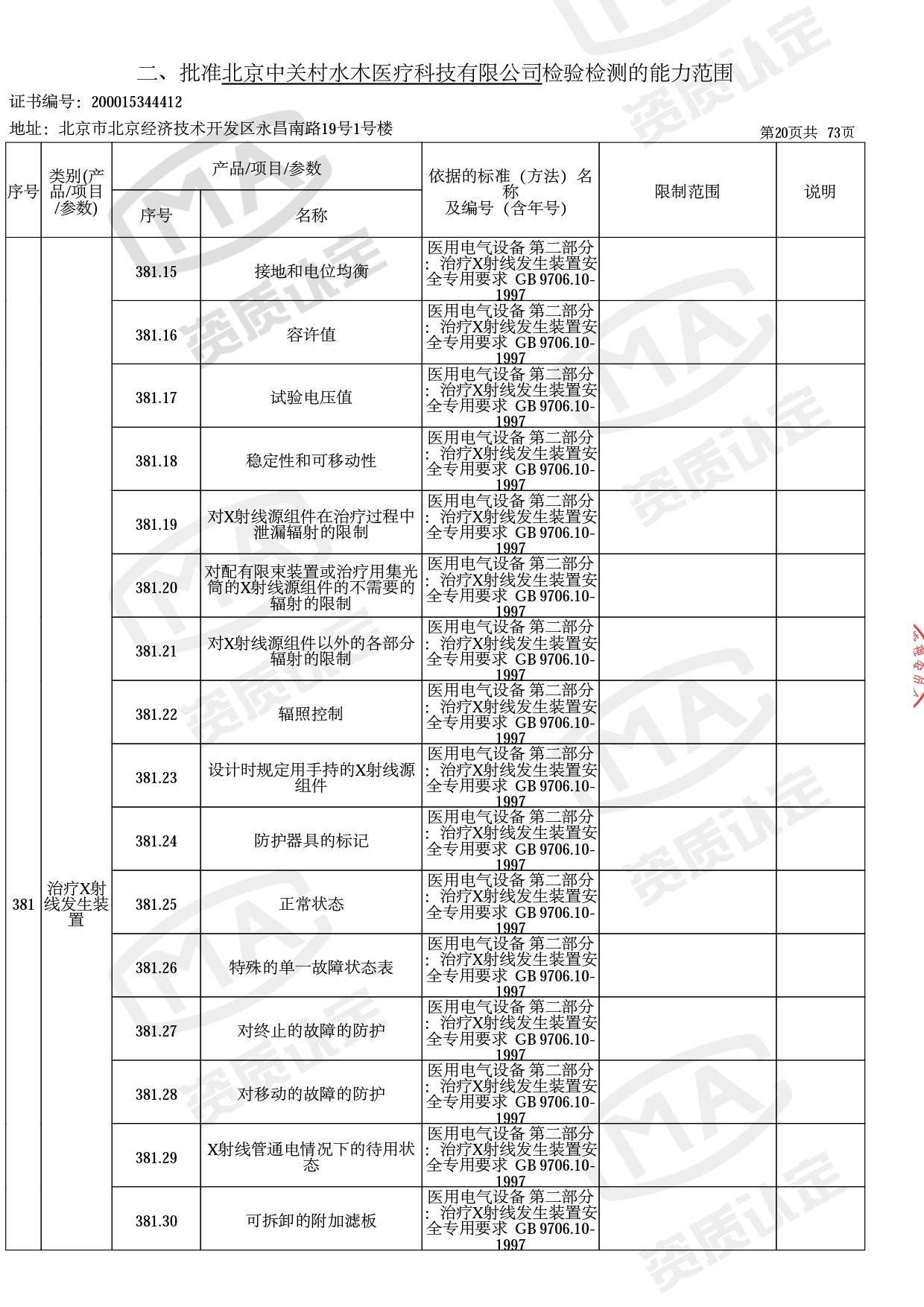
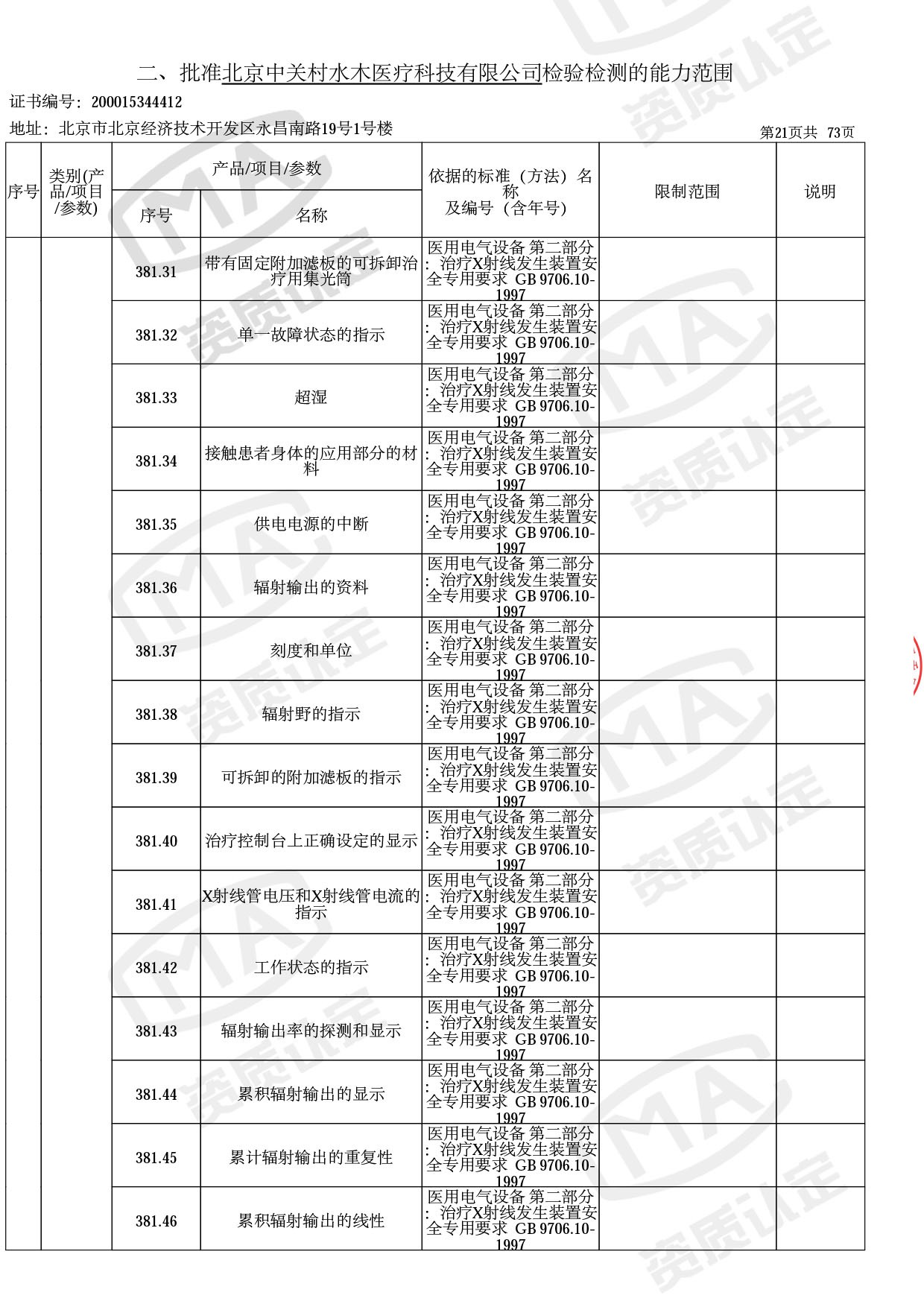
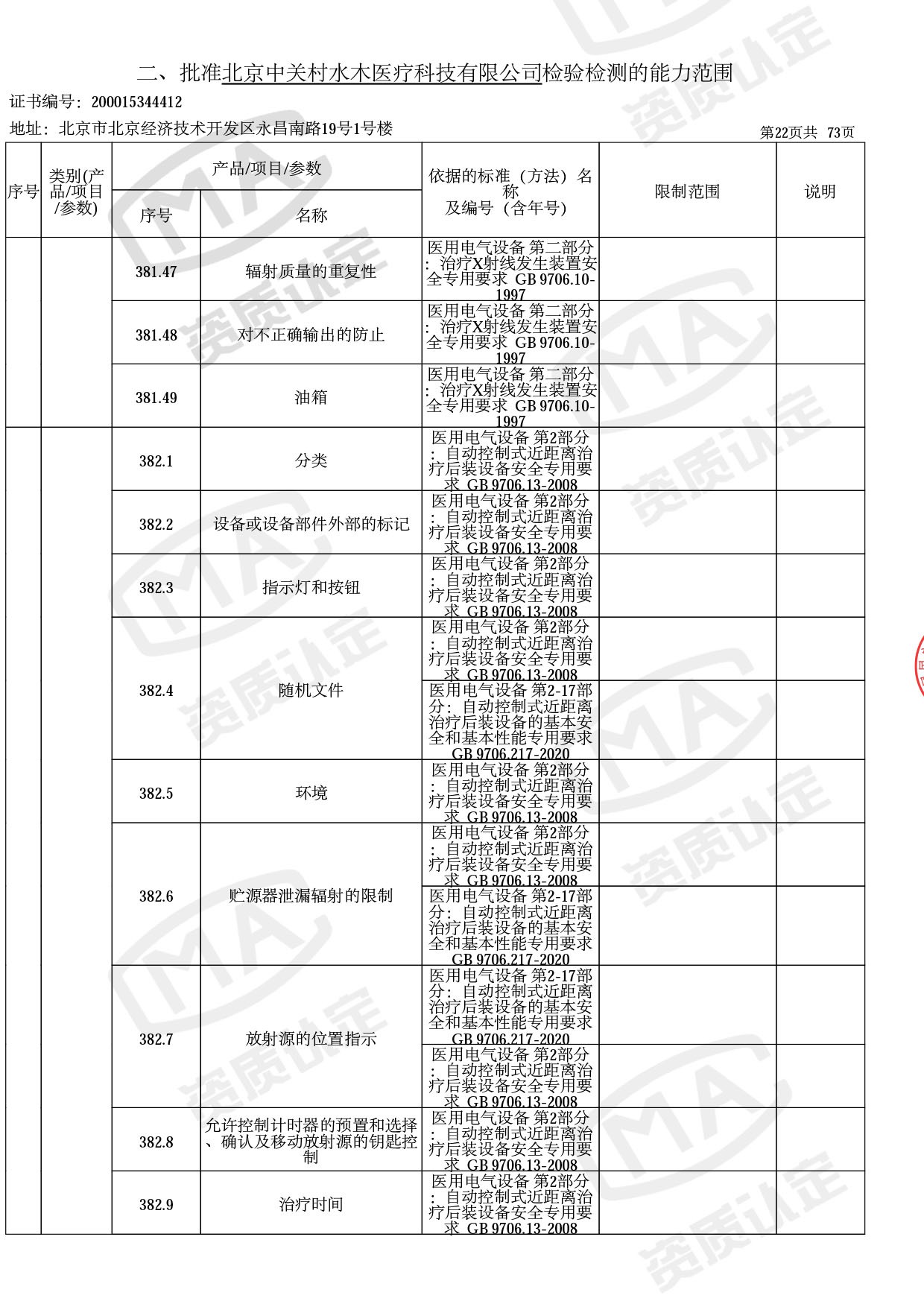
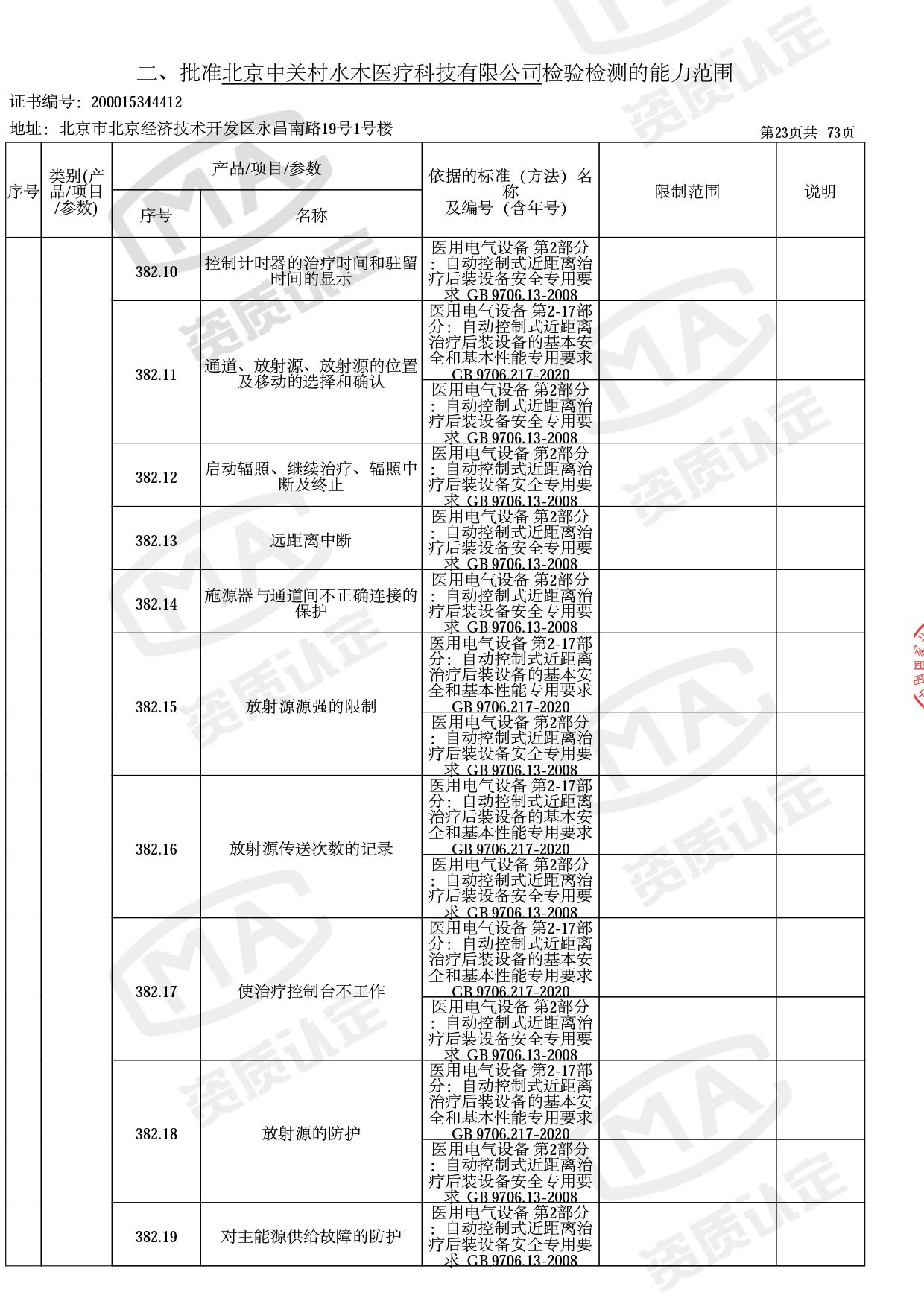
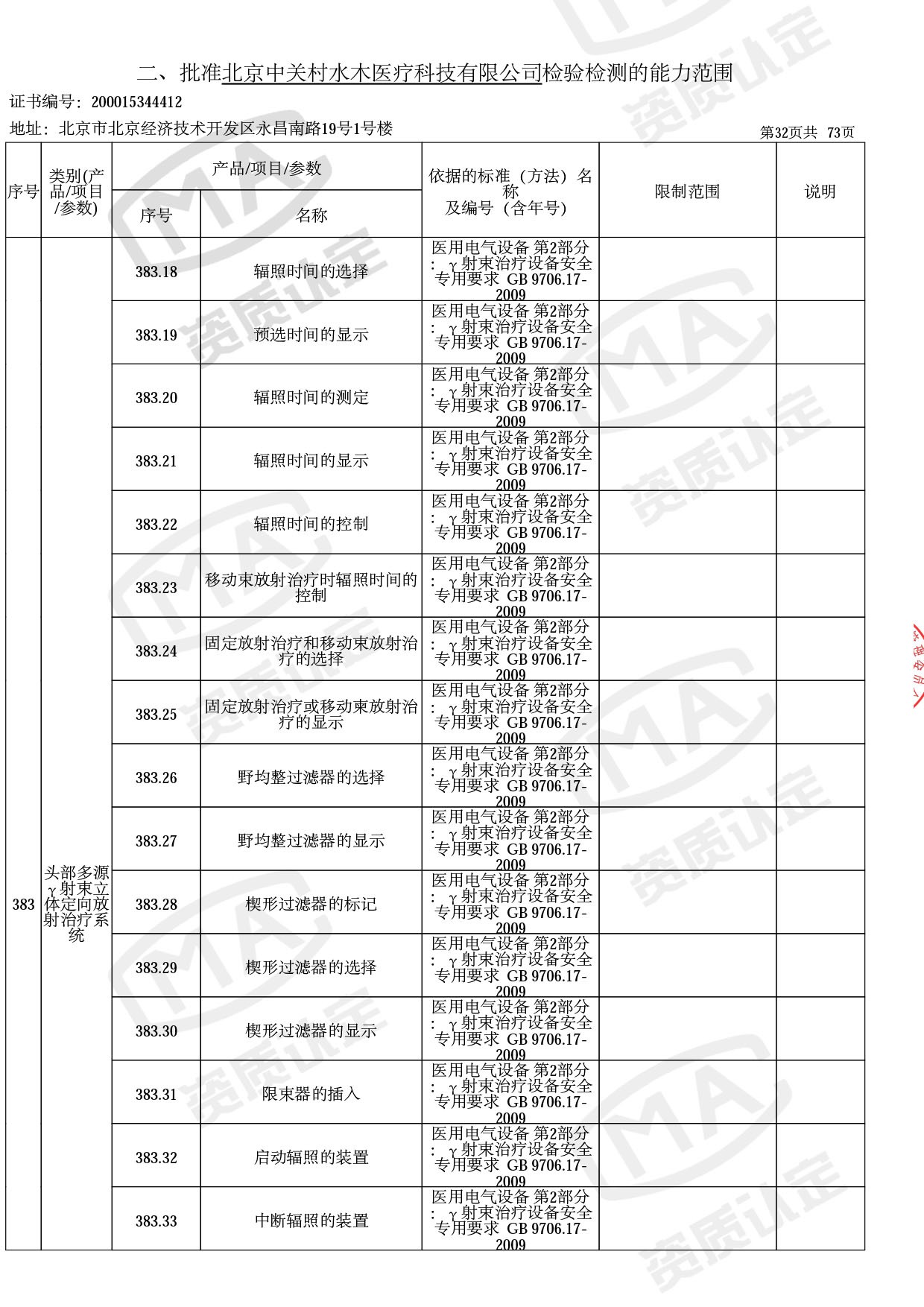
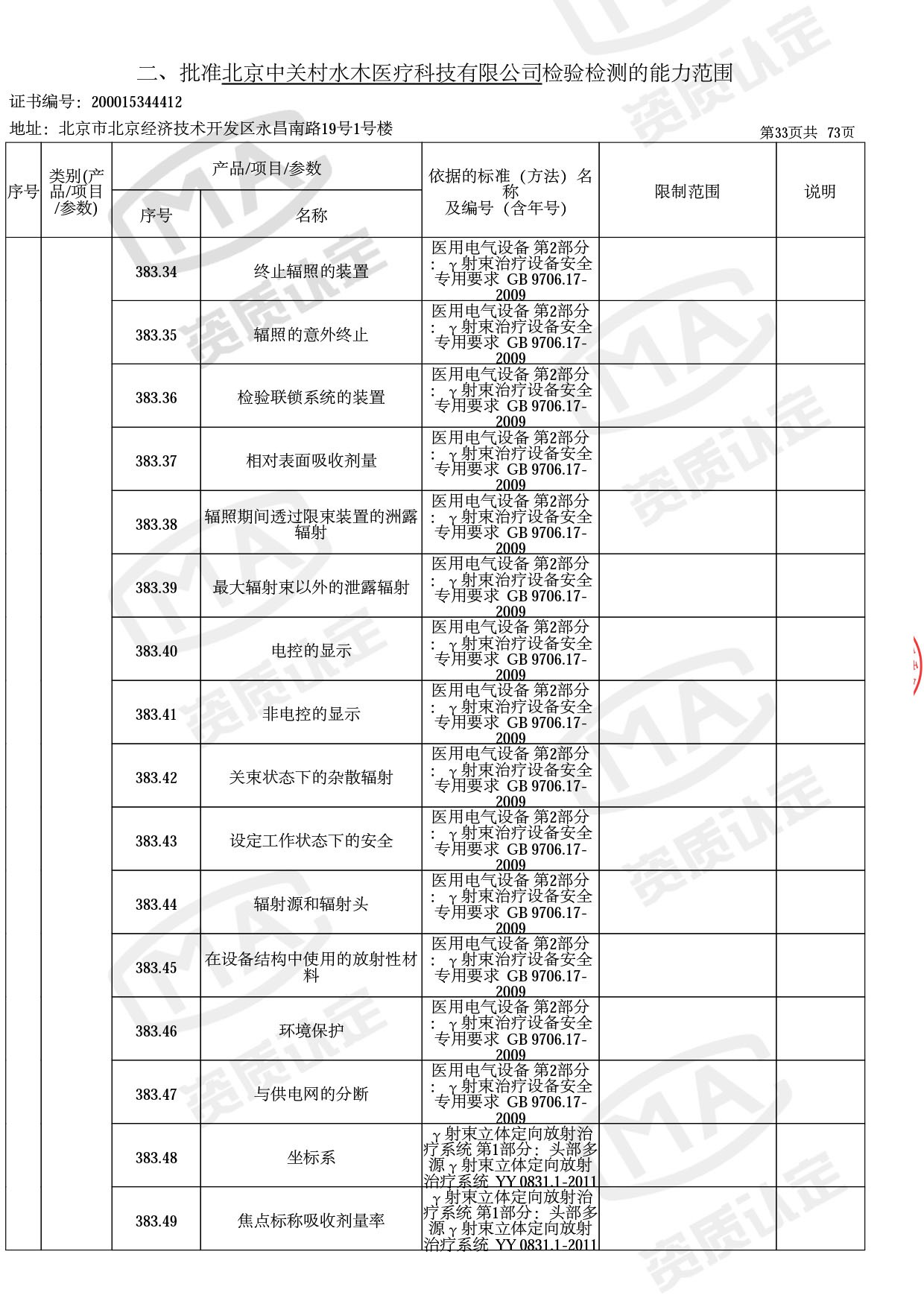
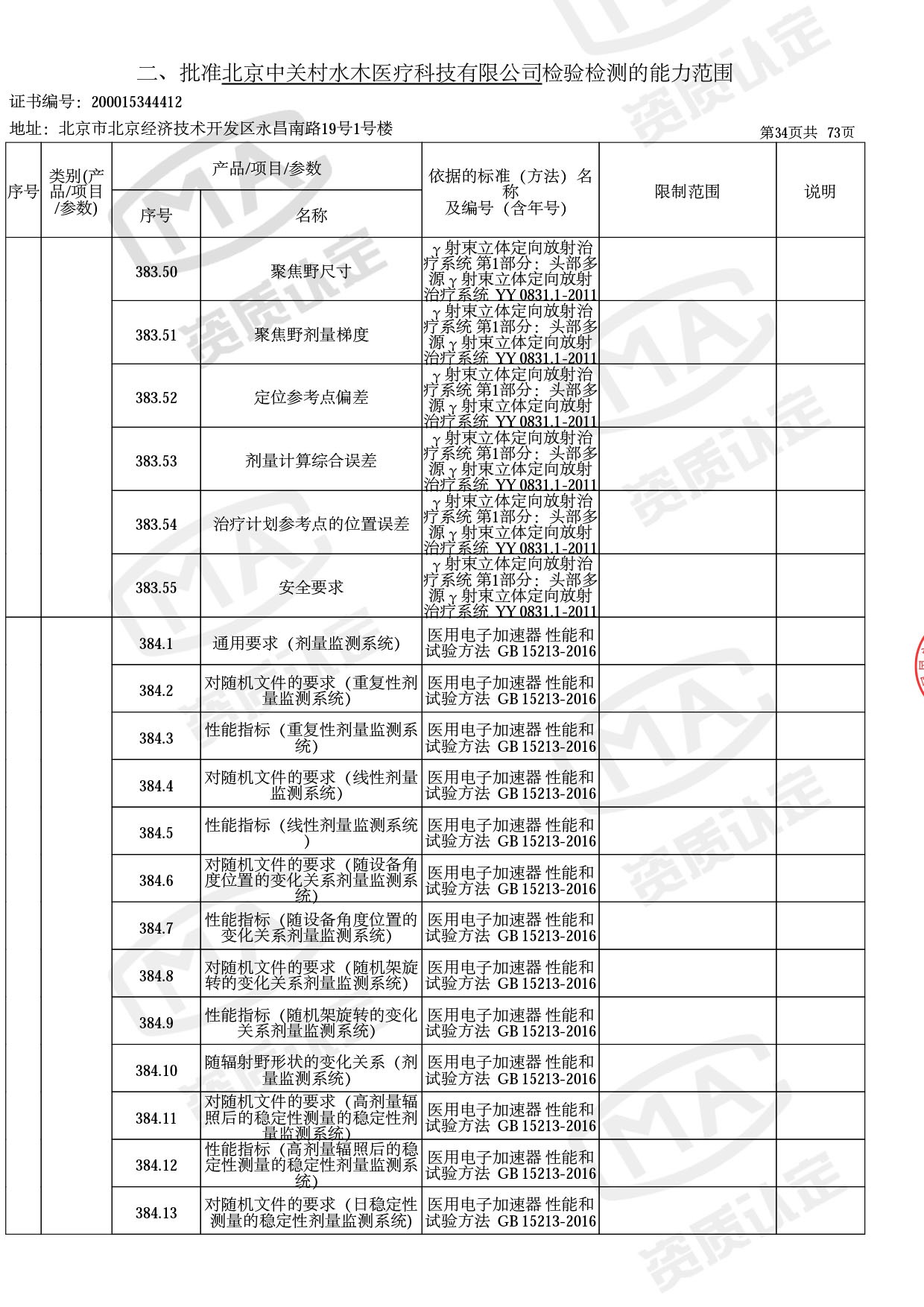
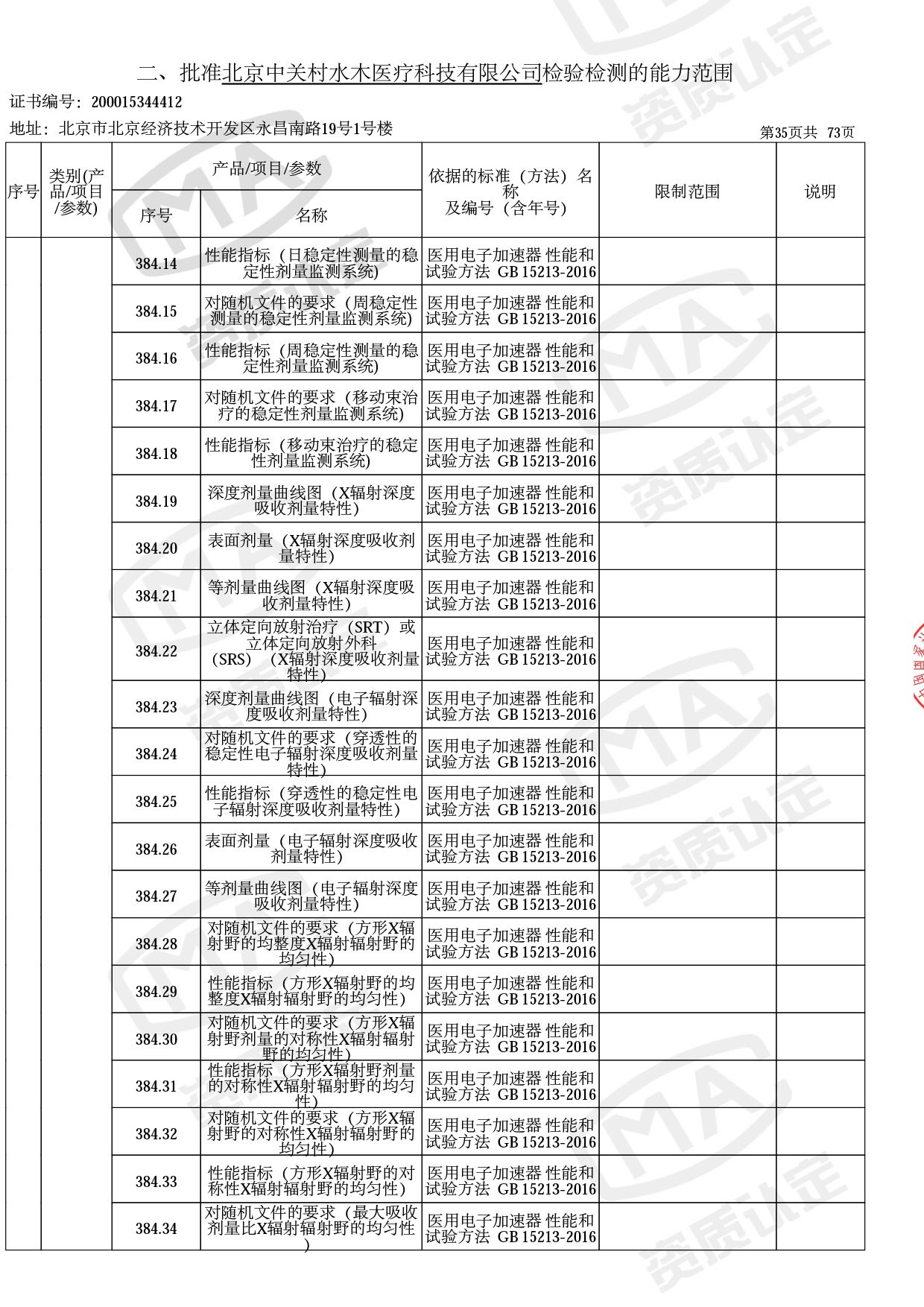
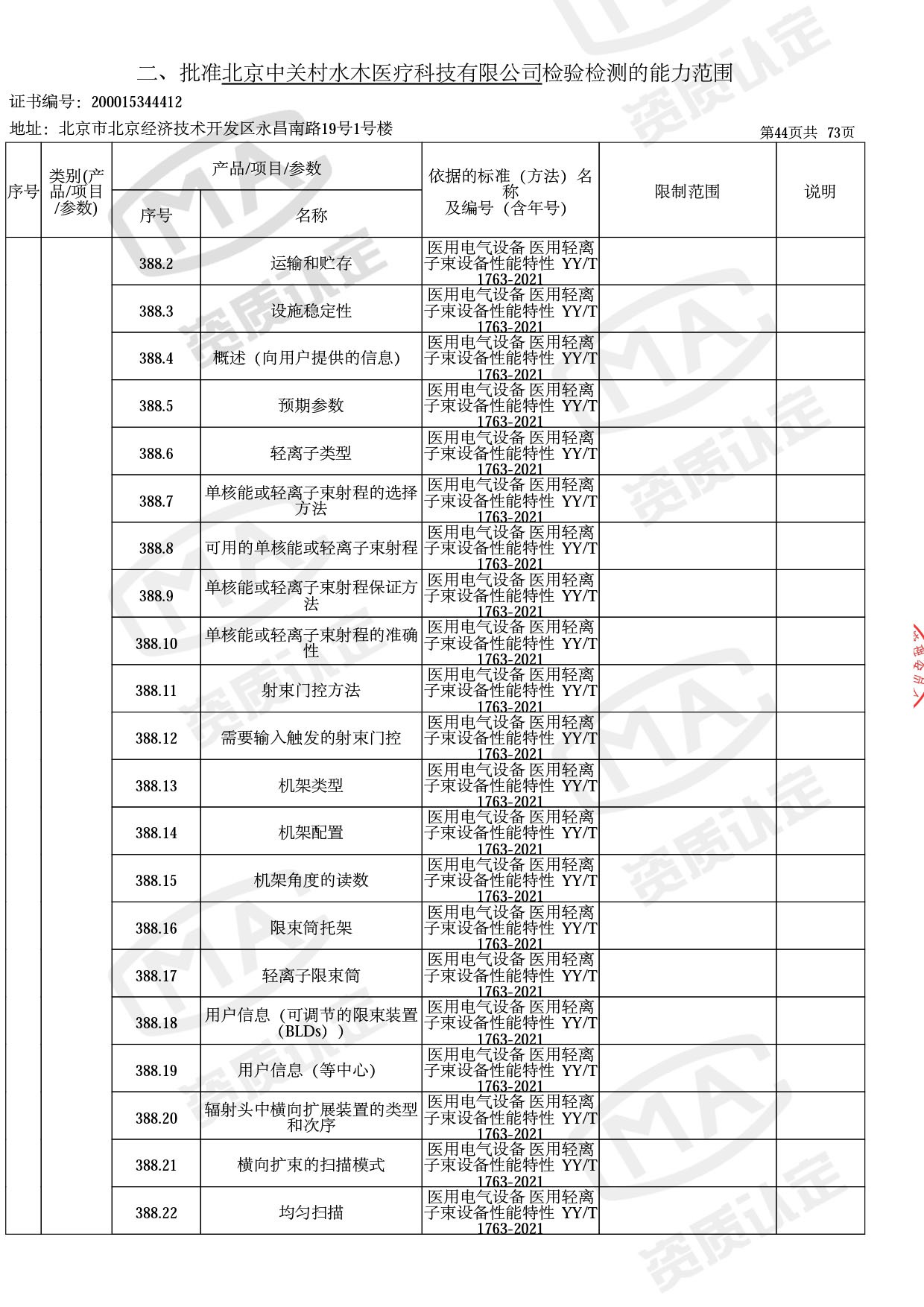
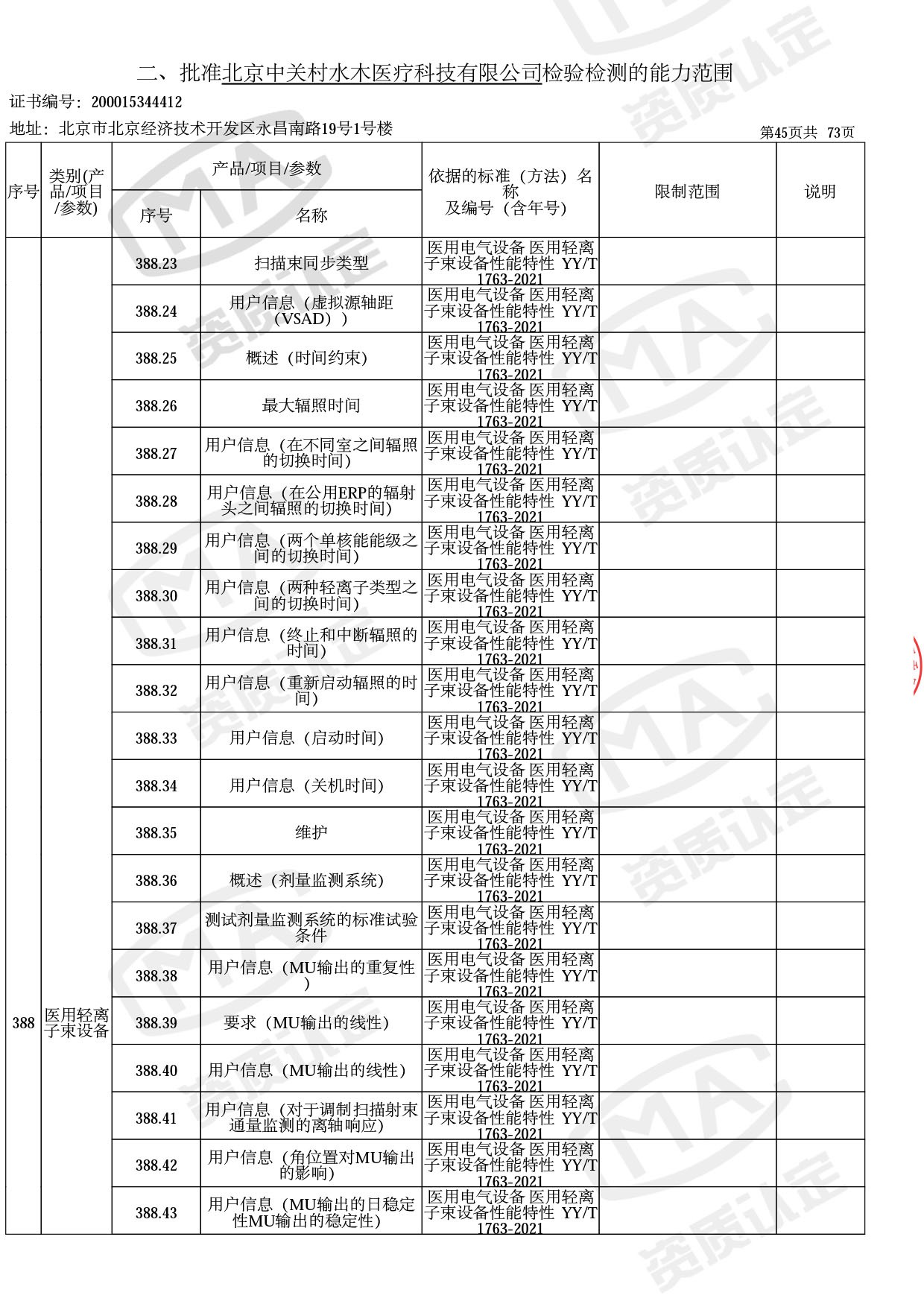
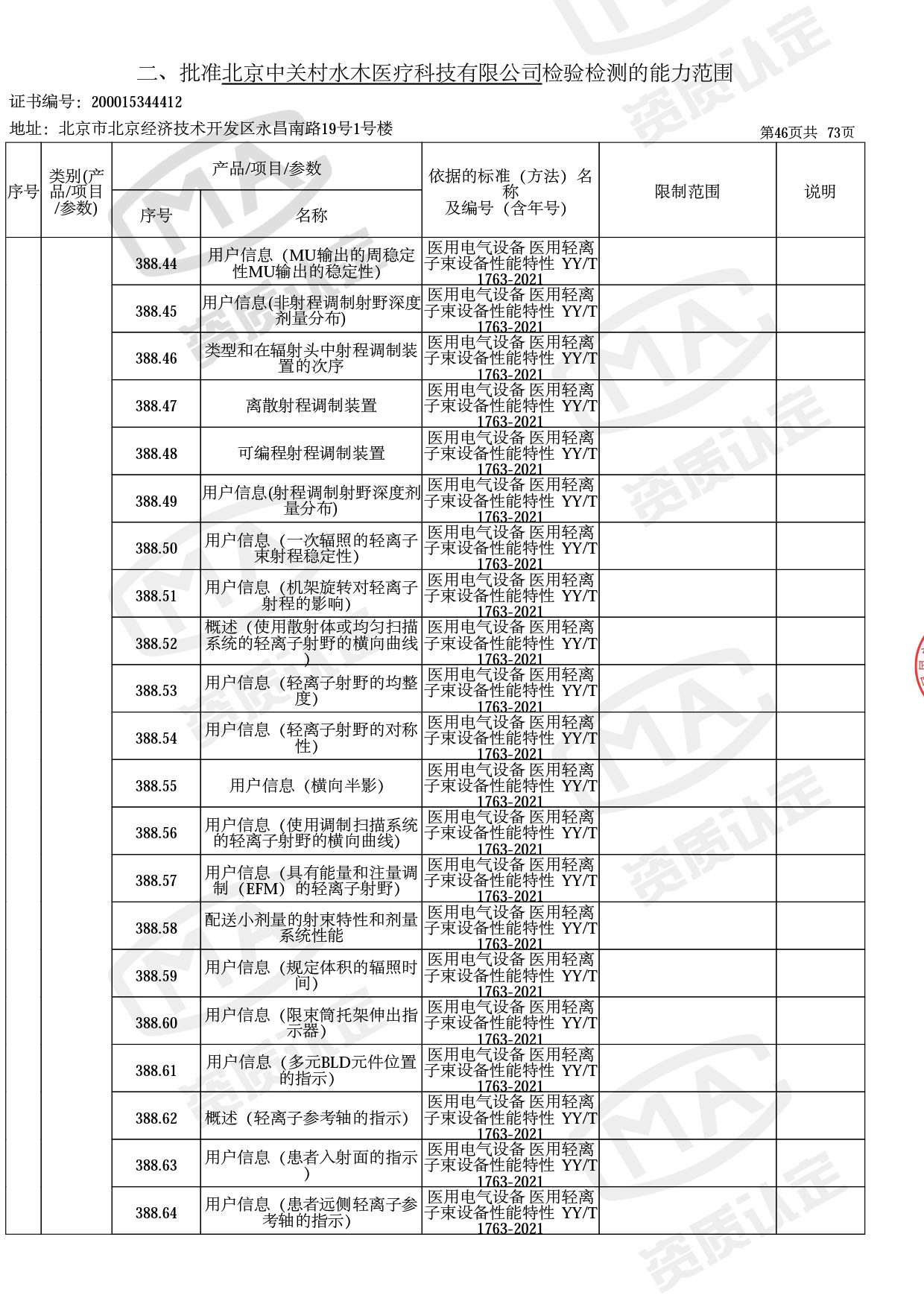
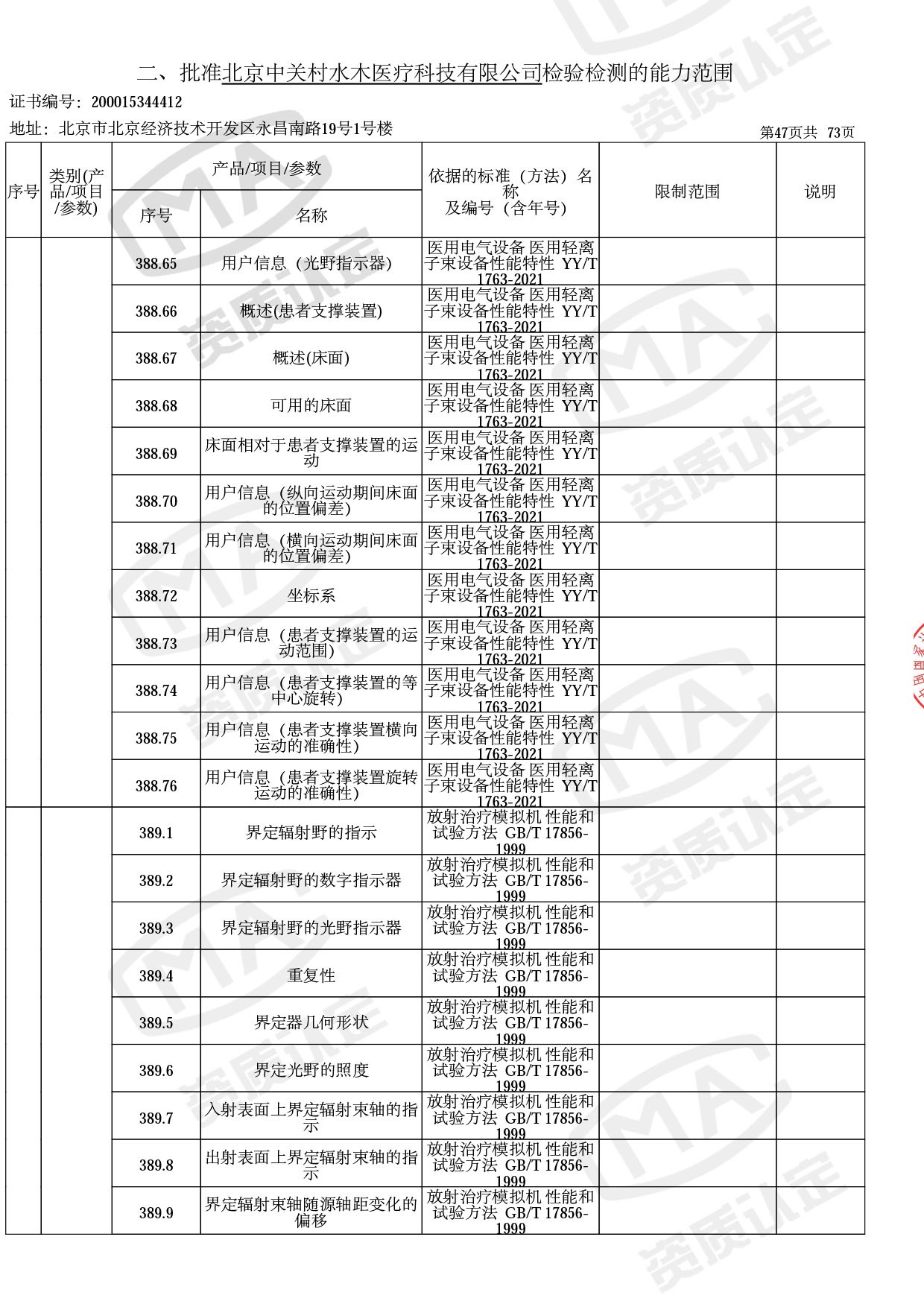
● 放疗类产品:
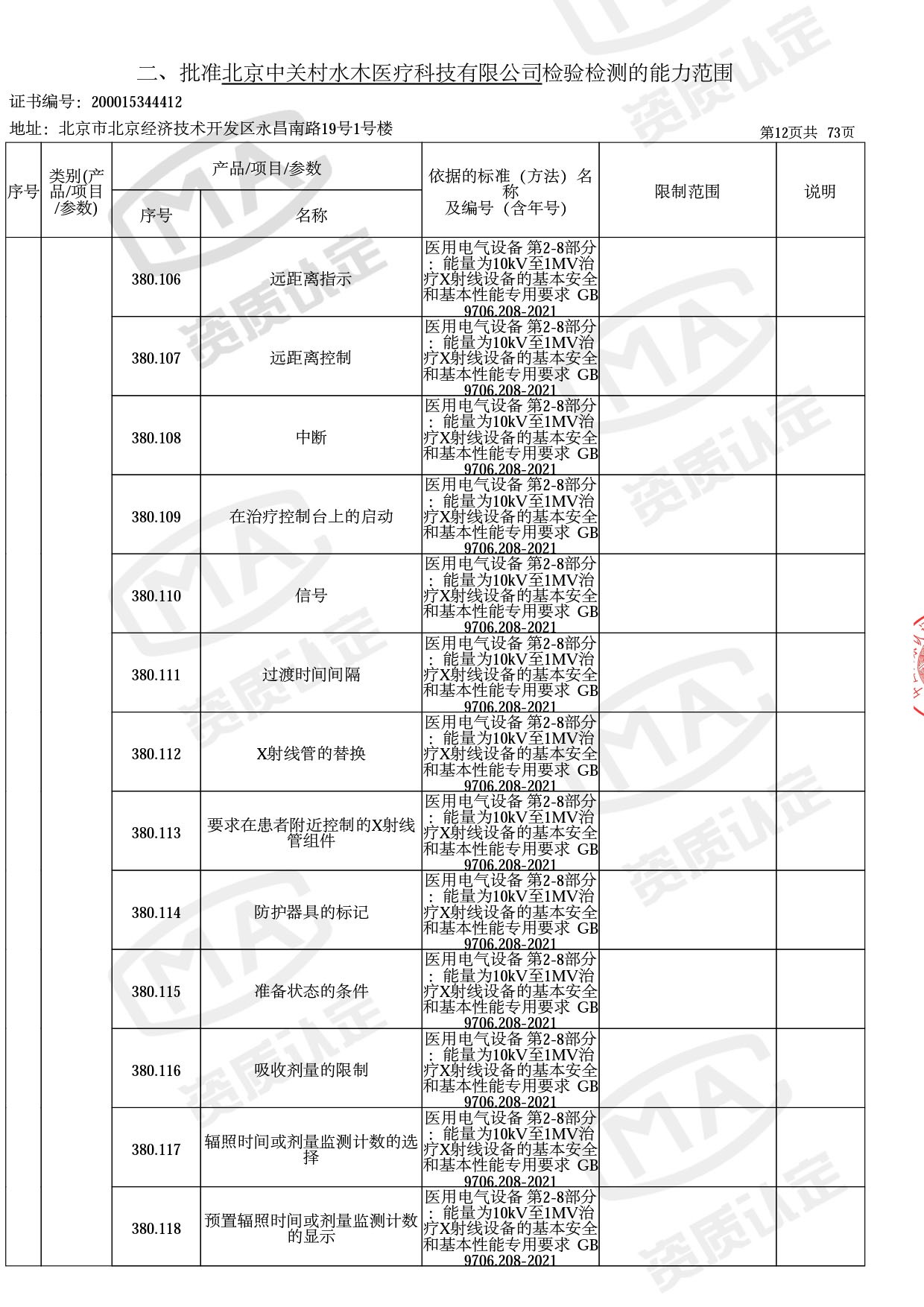
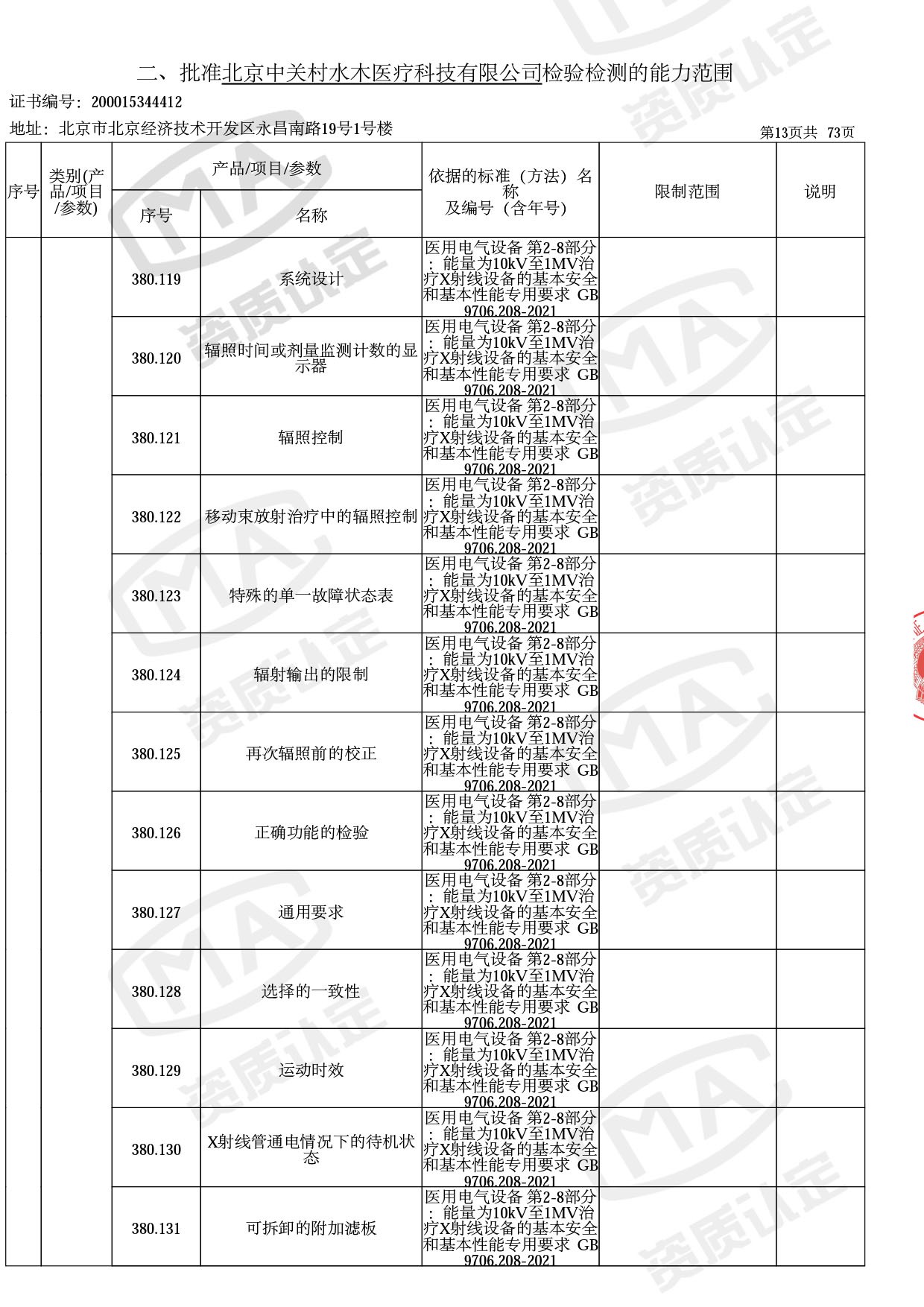
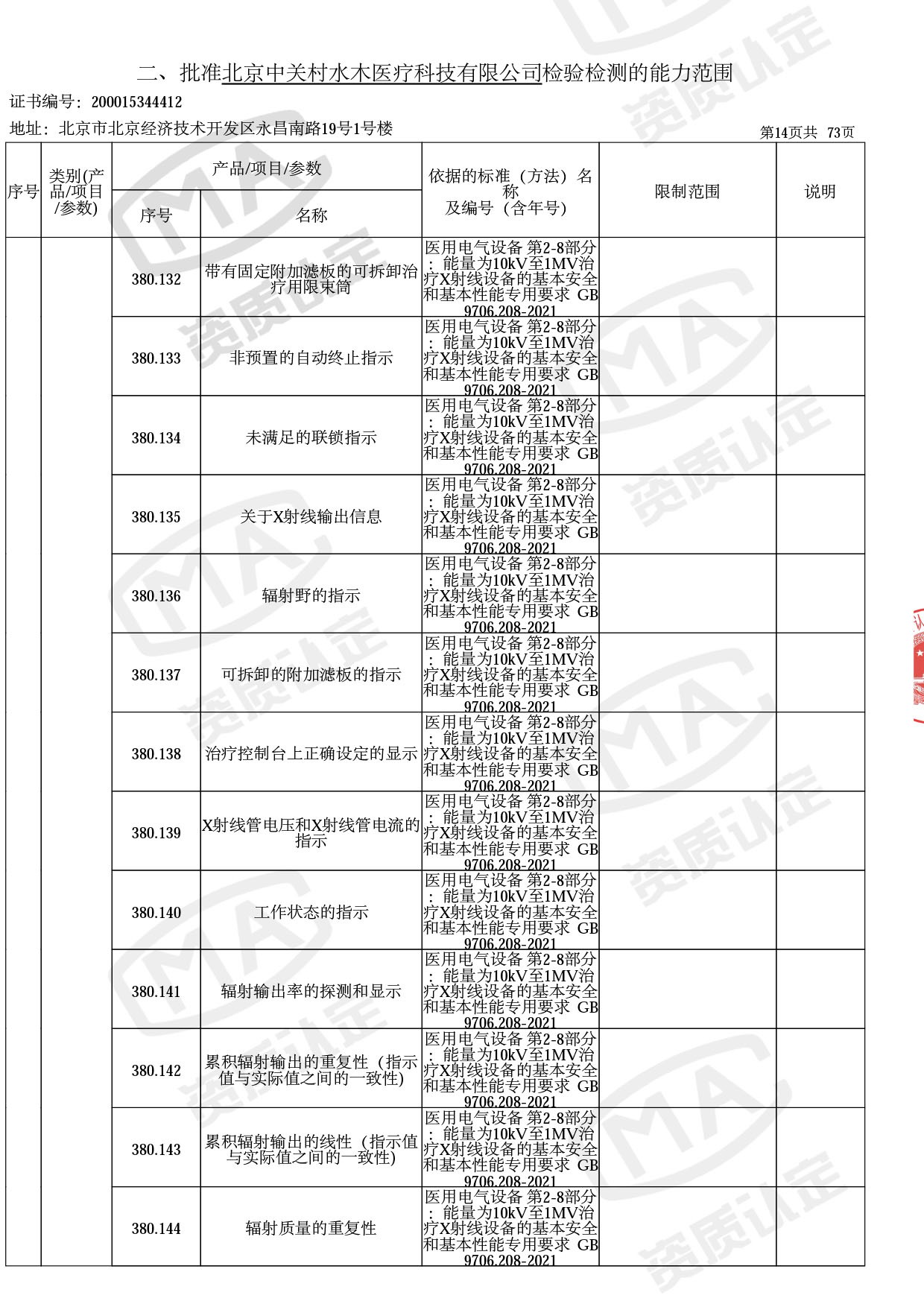
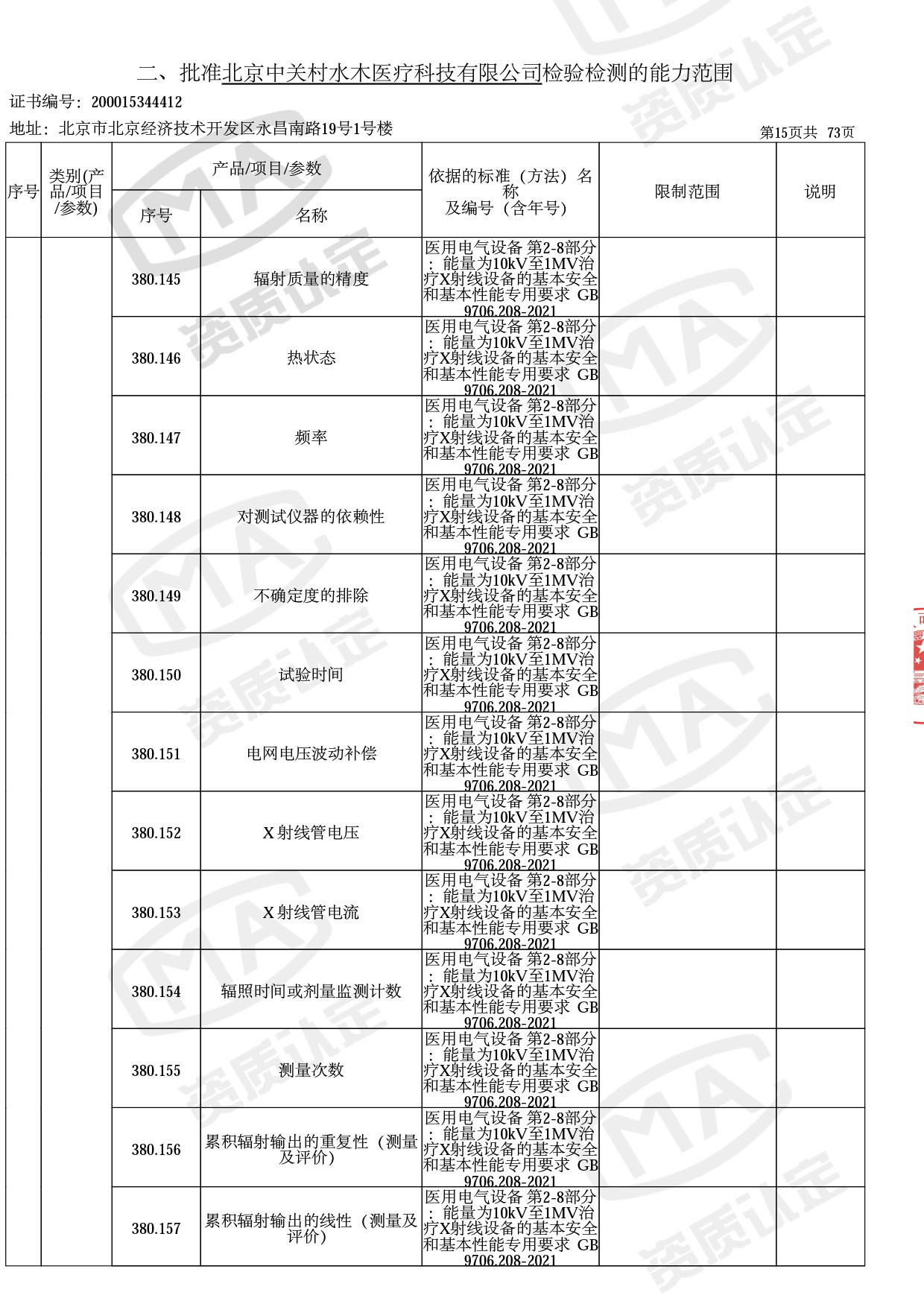
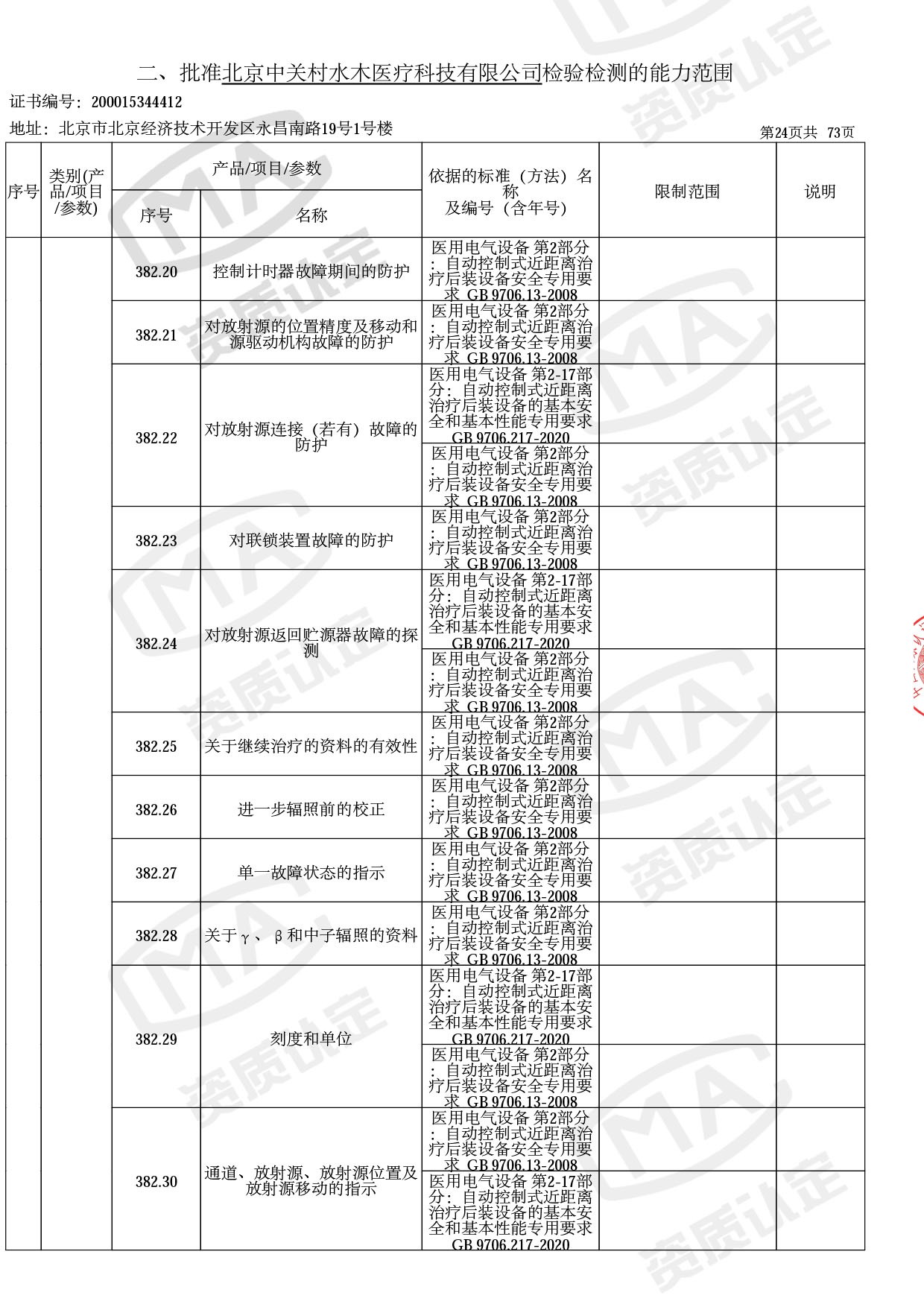
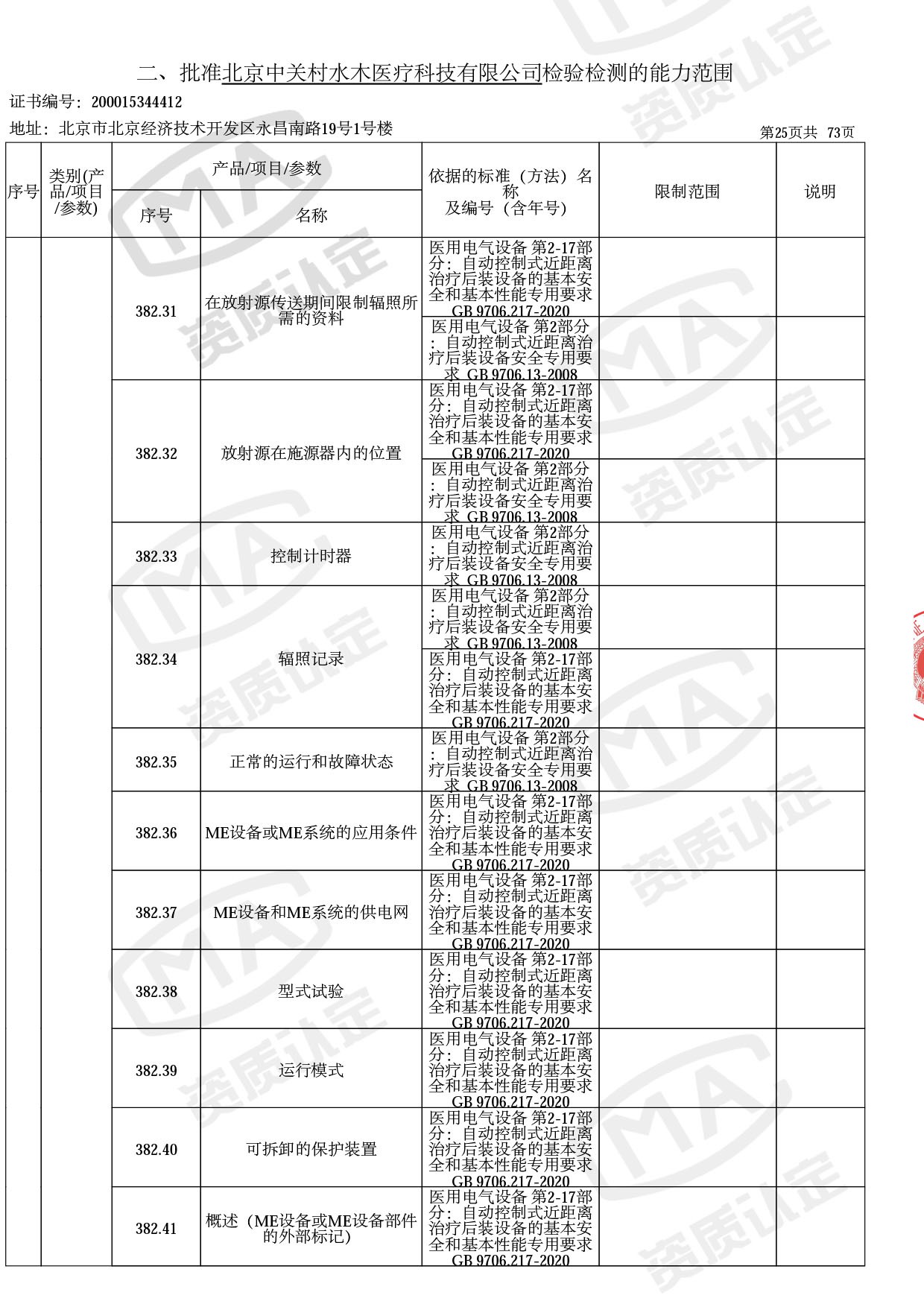
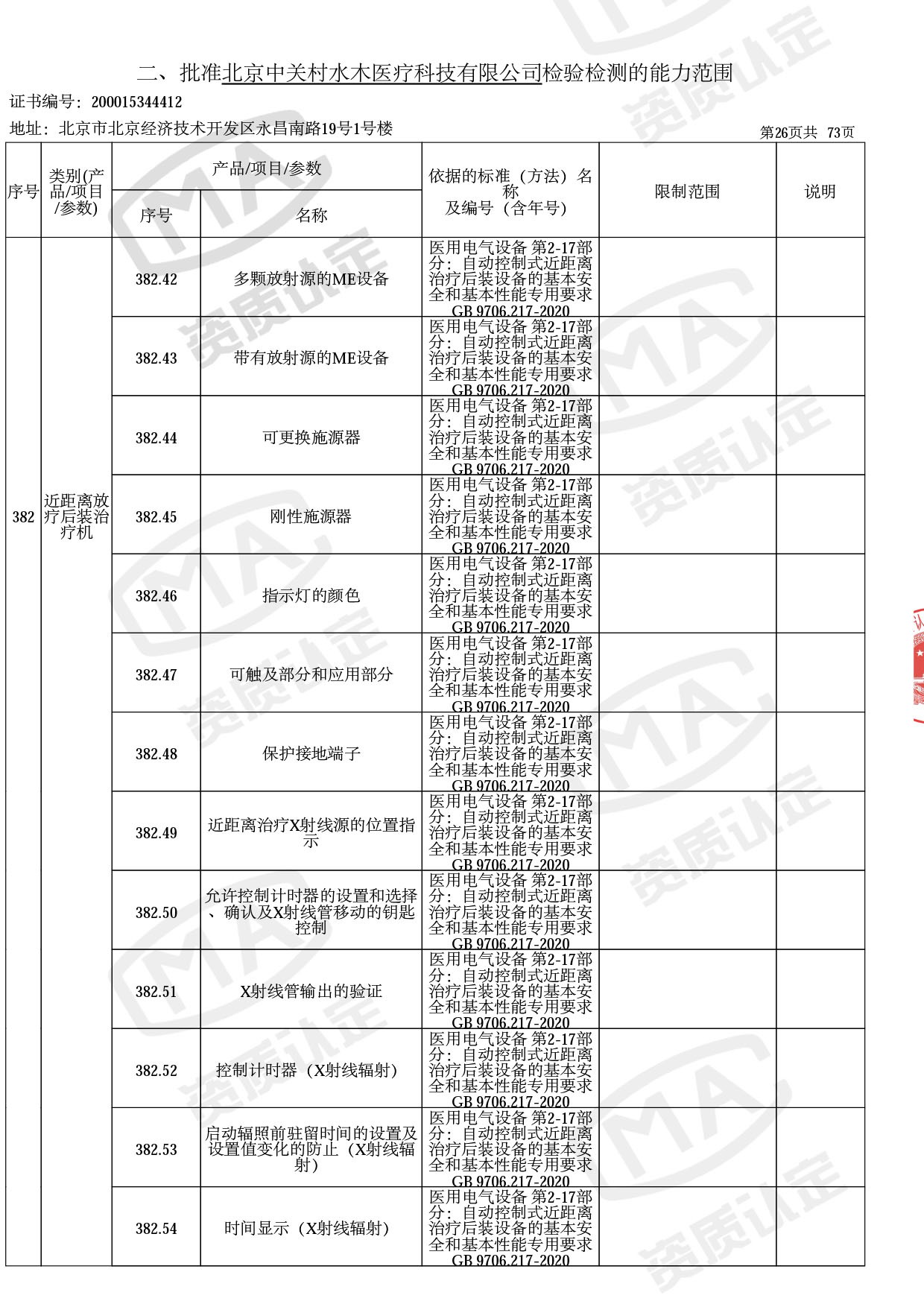
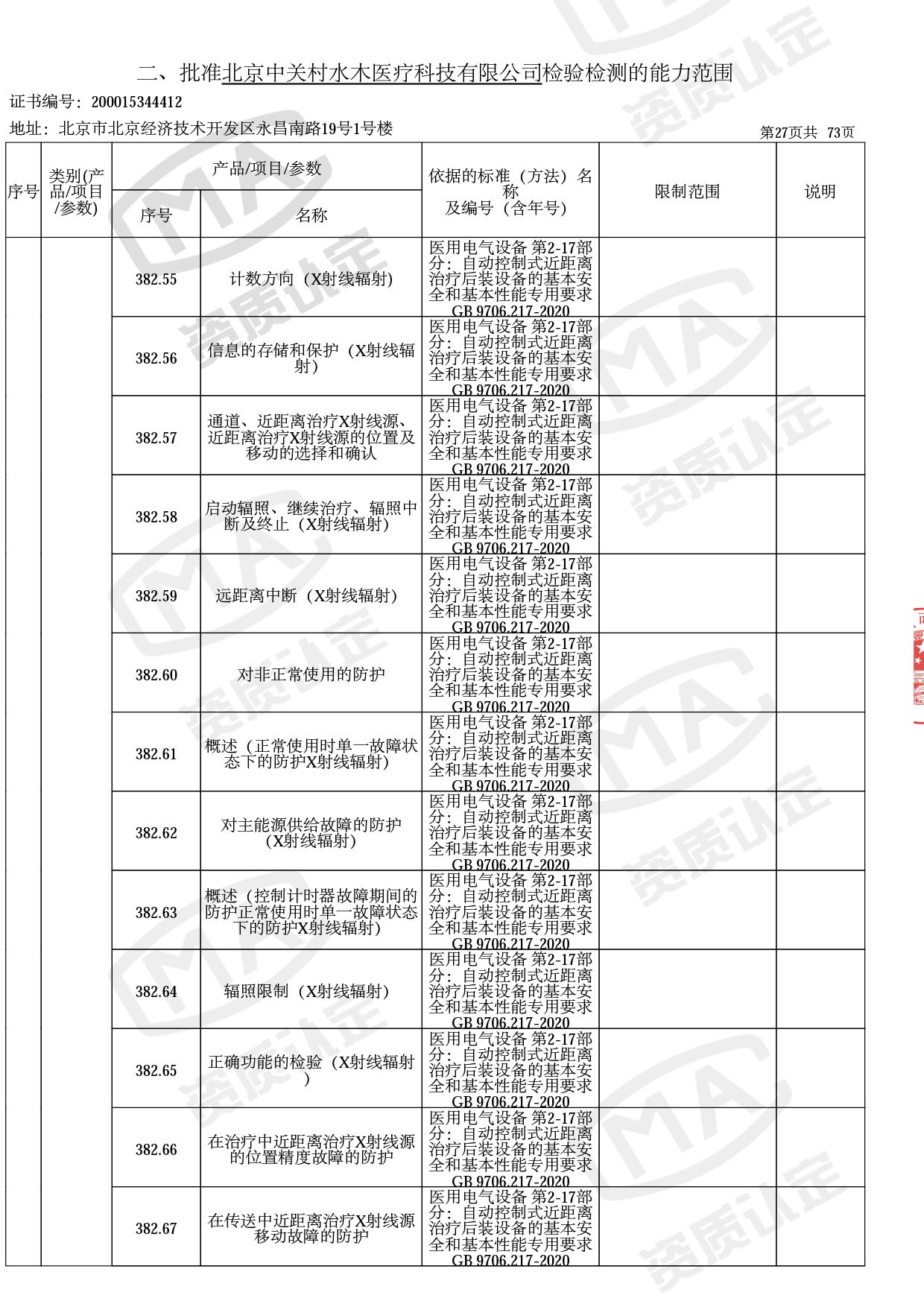
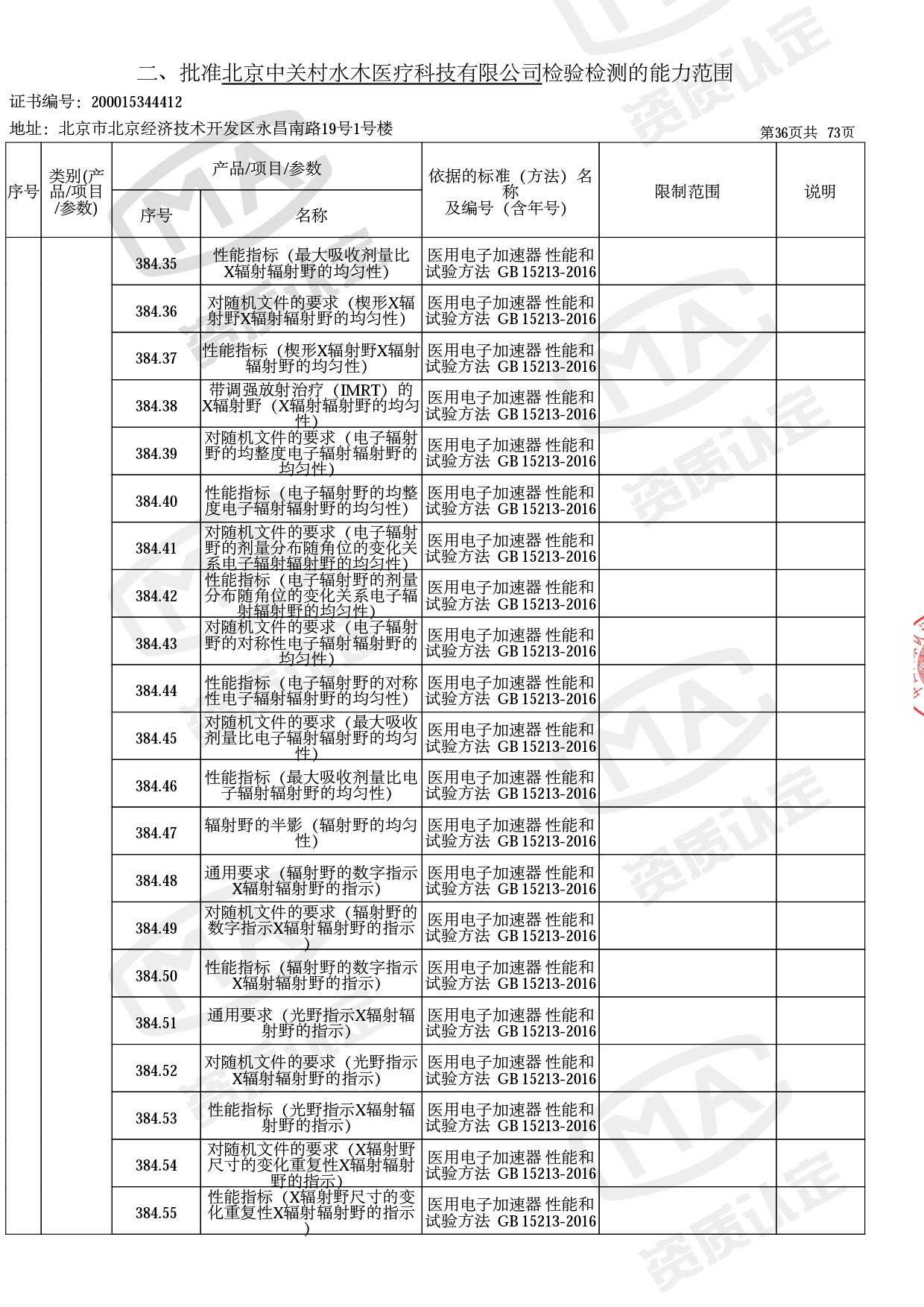
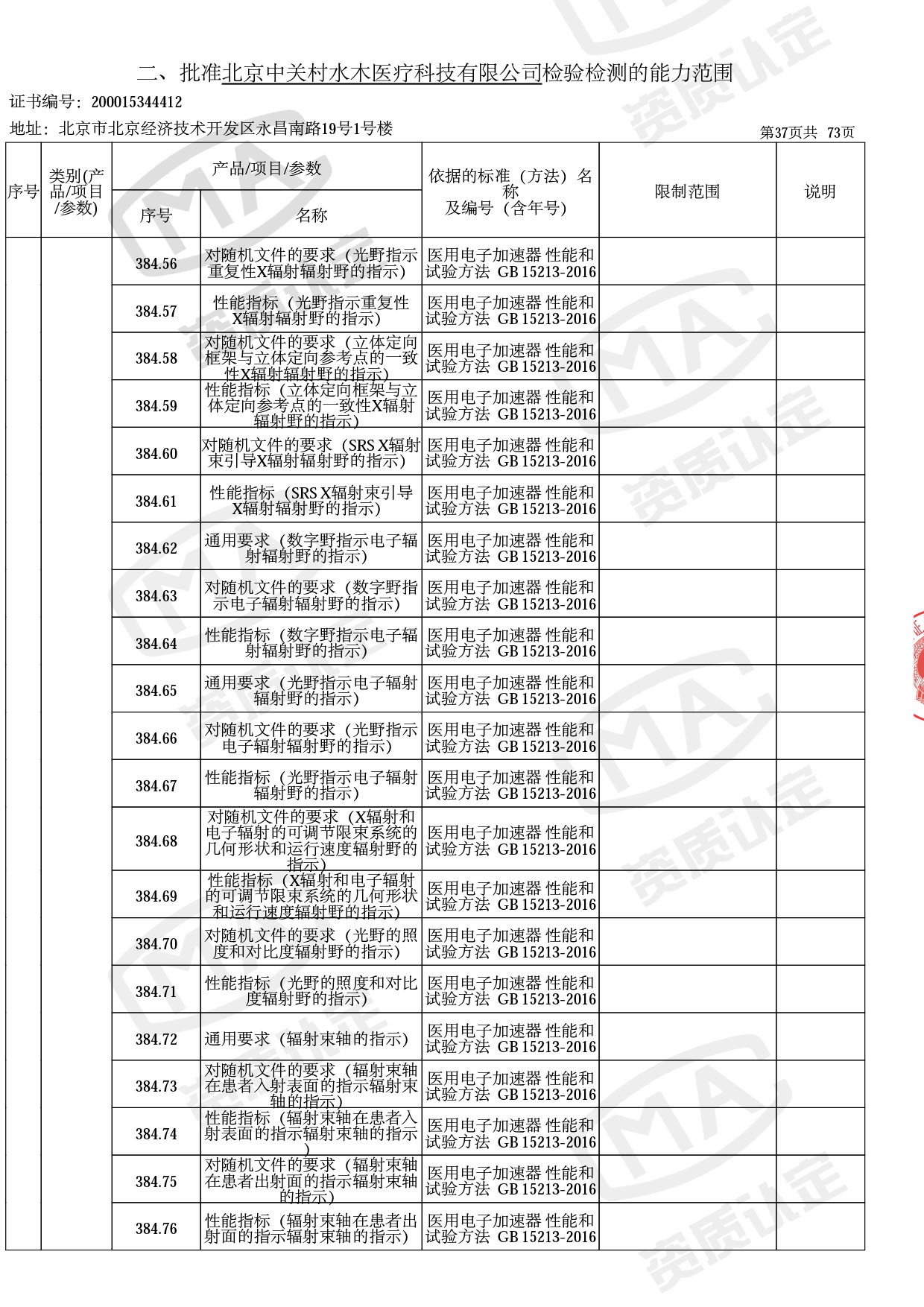
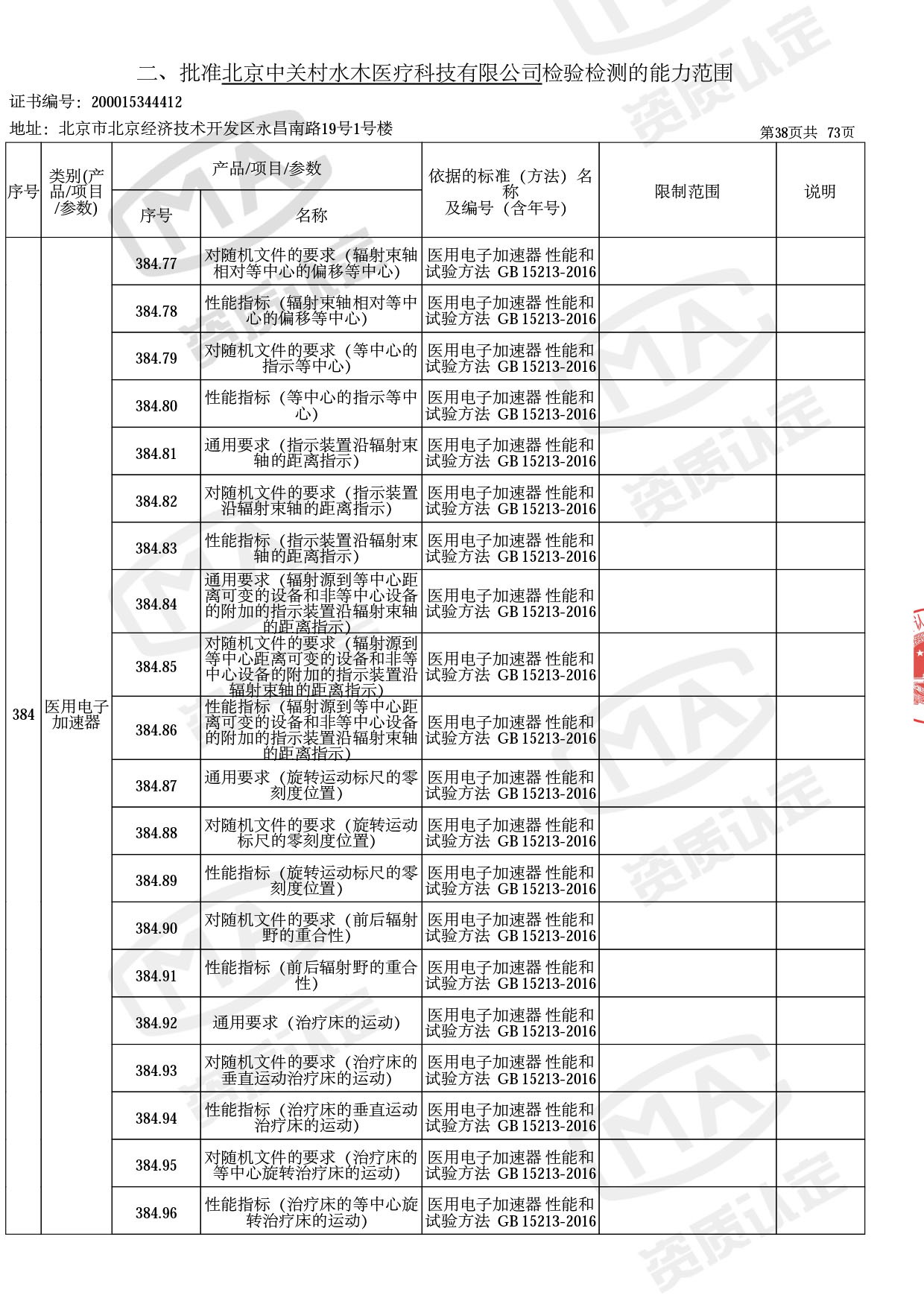
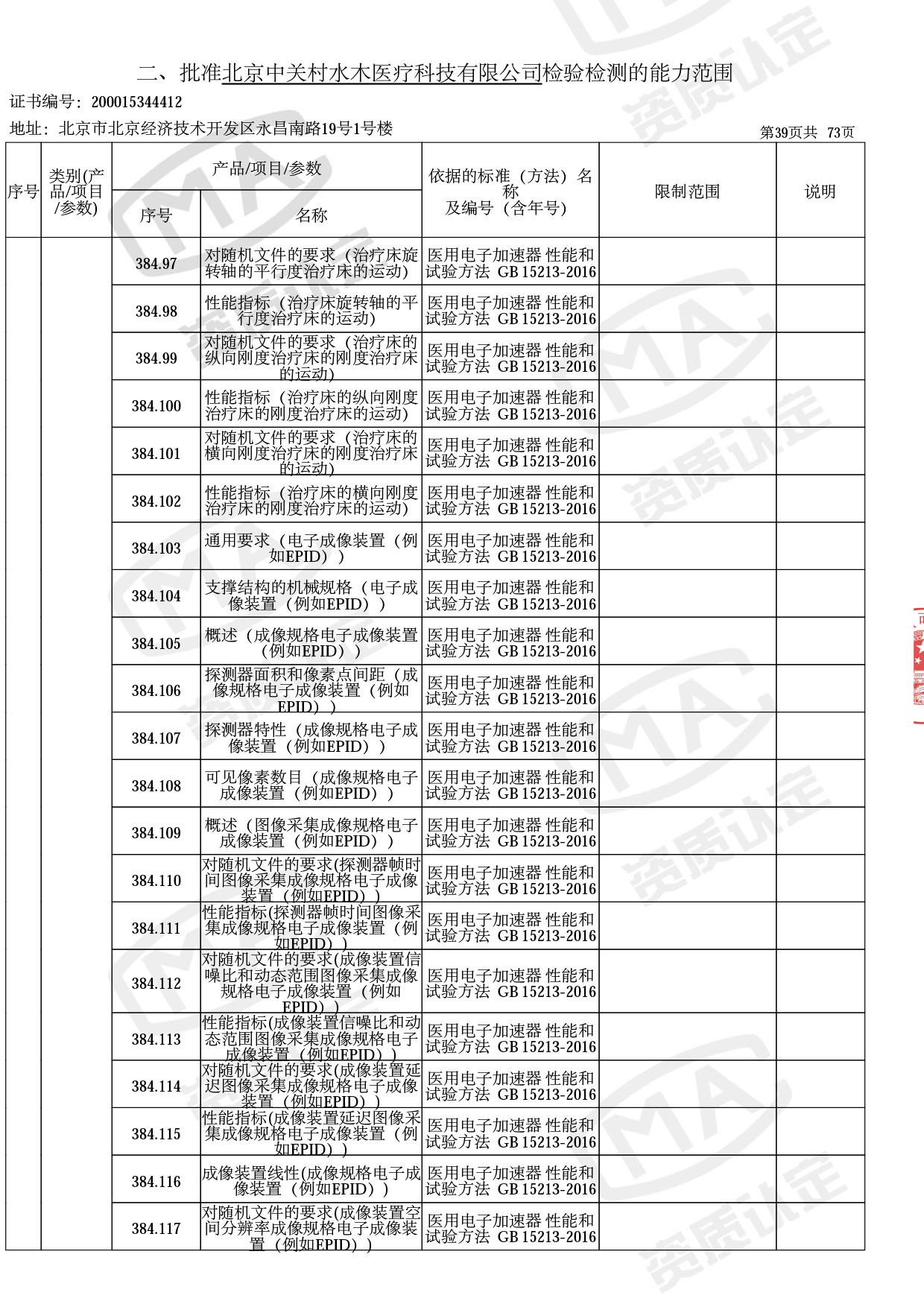
医用电子加速器性能和试验方法GB 15213-2016、医用电气设备 第2-1部分:能量为1MeV至50MeV电子加速器基本安全和基本性能专用要求GB 9706.201-2020;射线专用标准方法:医用治疗X射线机通用技术条件 YY/T 0317-2007,X射线图像引导放射治疗设备 性能和试验方法YY 1650-2019;
● 网络安全标准方法:
医用电气设备网络安全基本要求YY/T 1843-2022等检测方法,共49个检测方法,1253个检测项目。
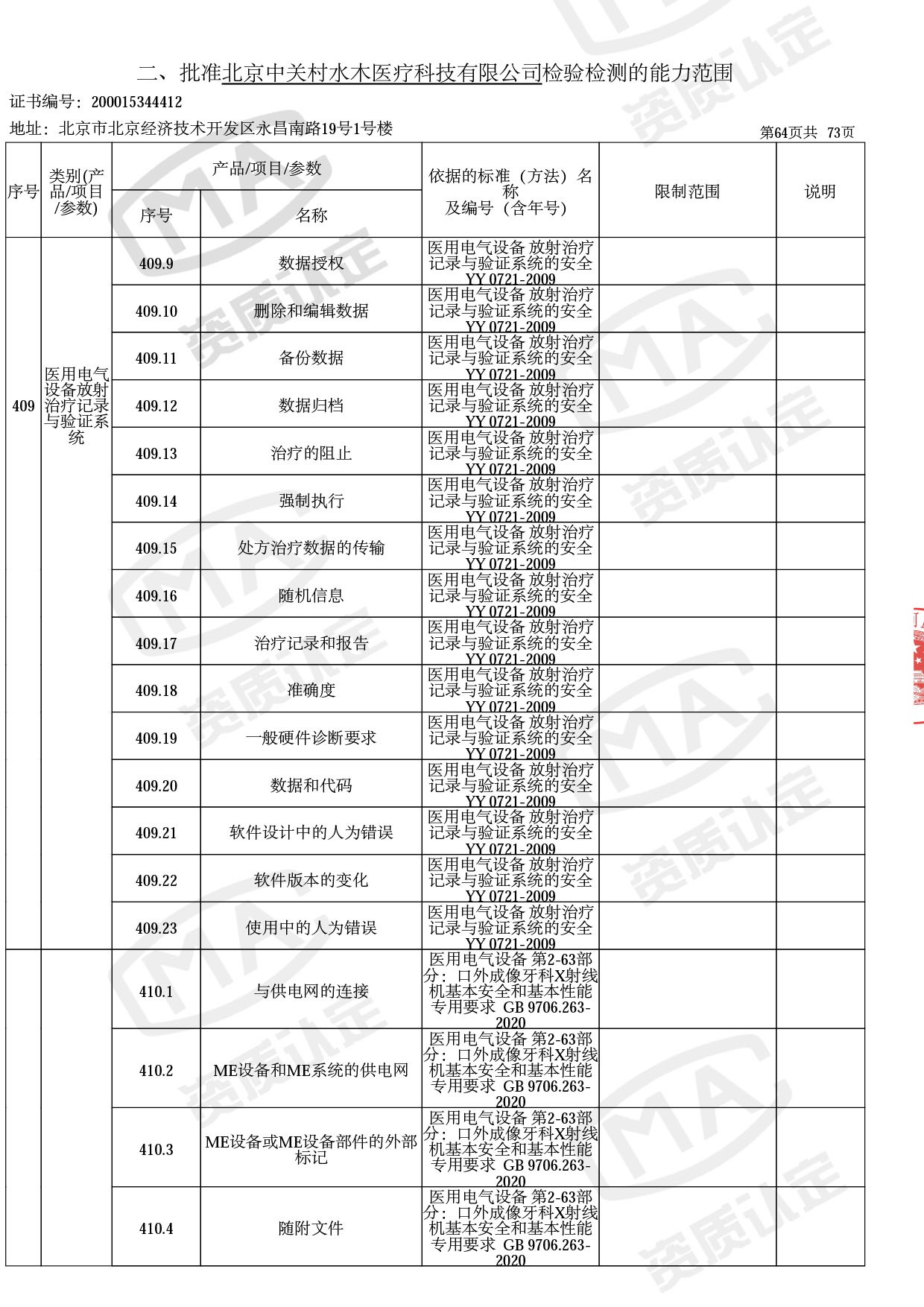
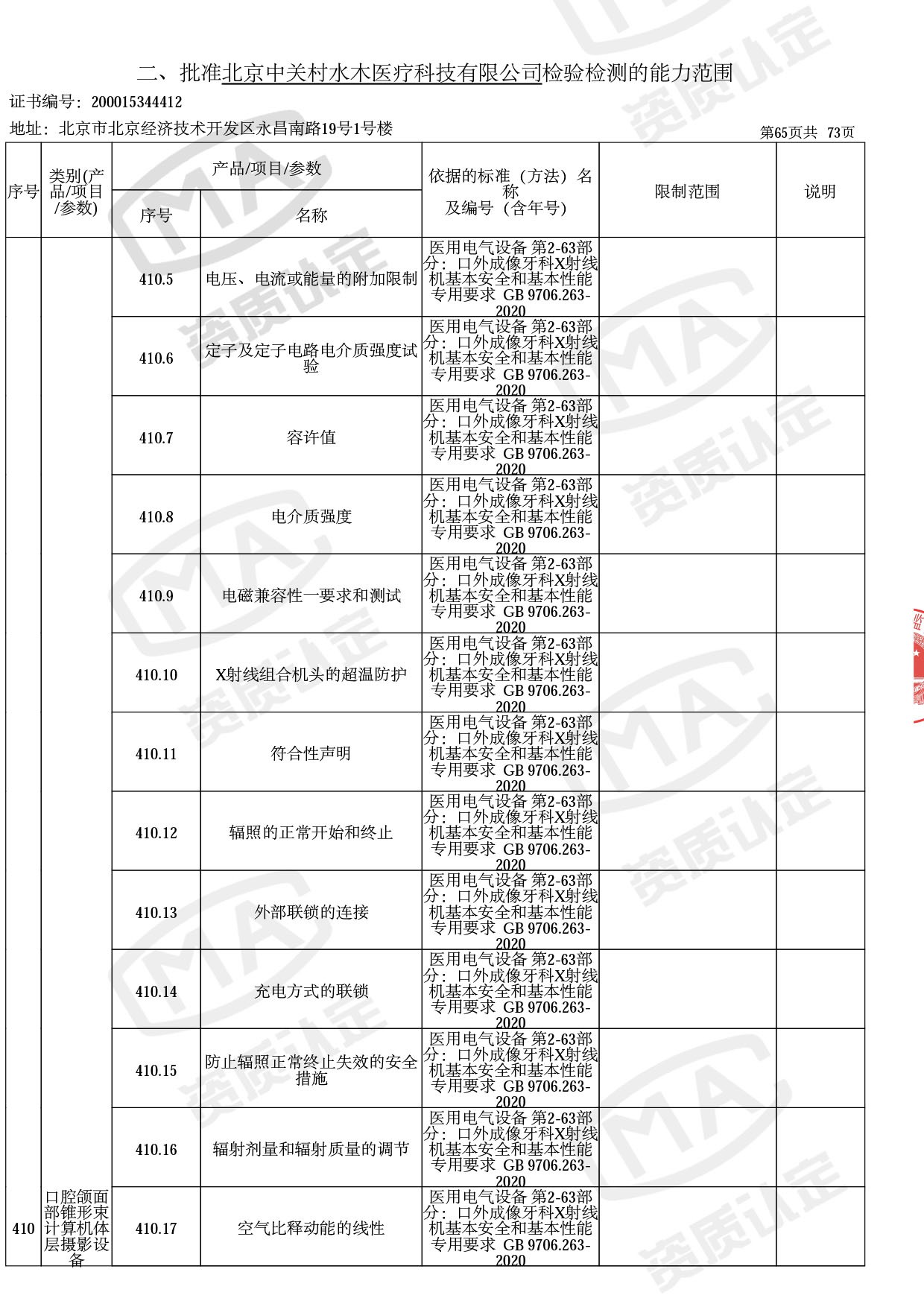
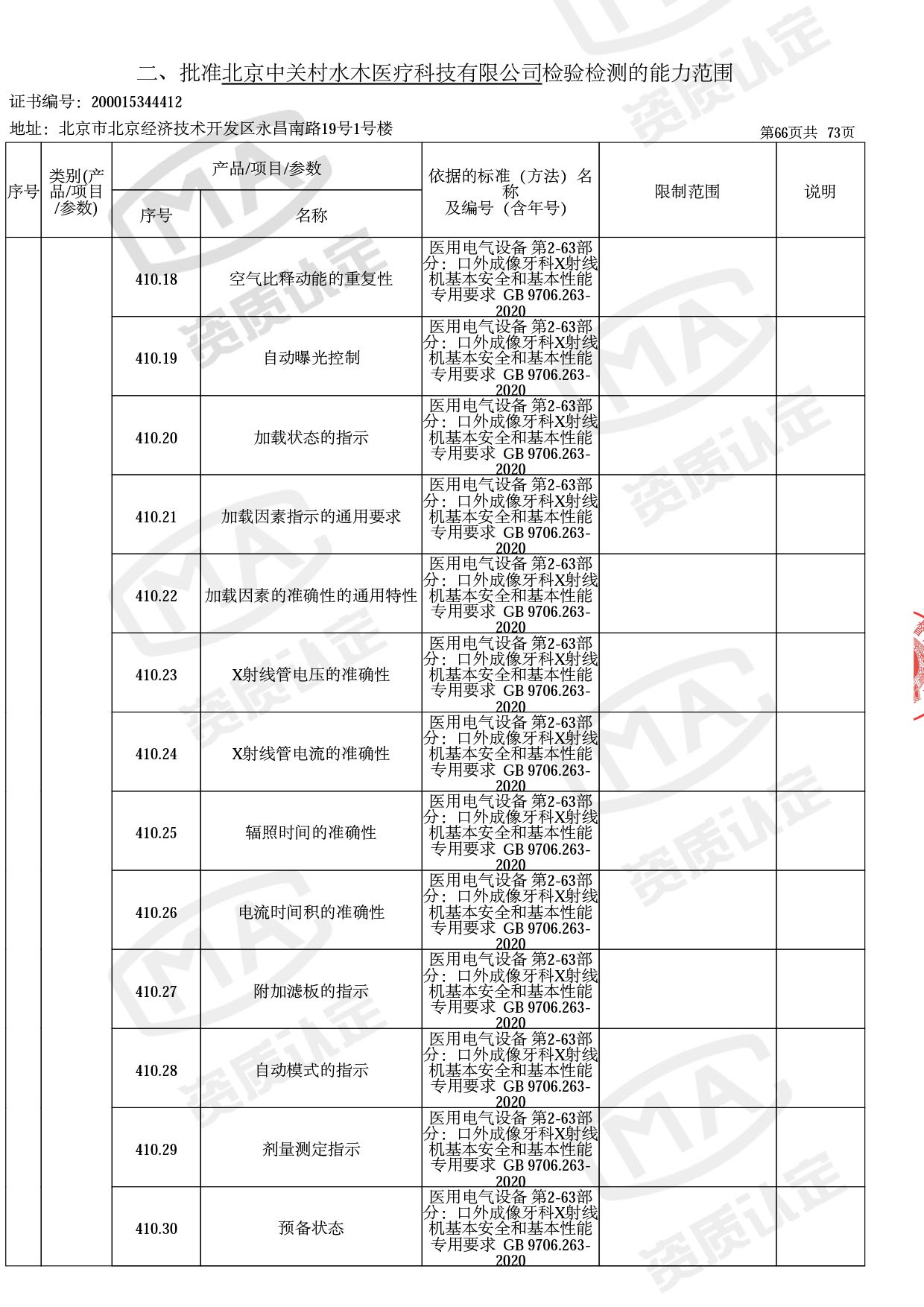
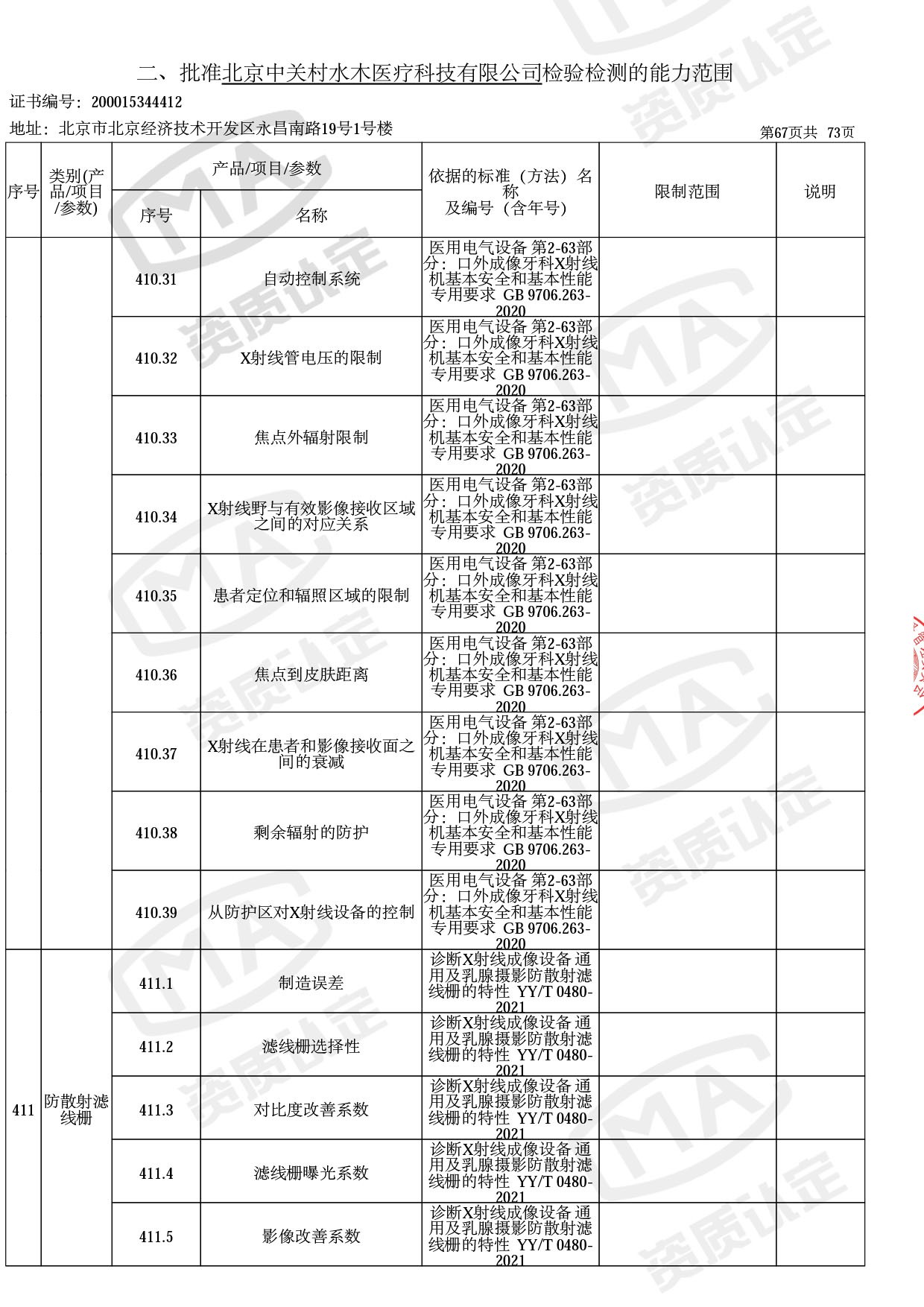
截止目前,我司获得CMA资质标准方法共计694个,8278个检测项目。其中国家局发布的59项新版9706标准中,已获得资质标准方法46项,包含1个通用标准,6个并列标准及39个专用标准。
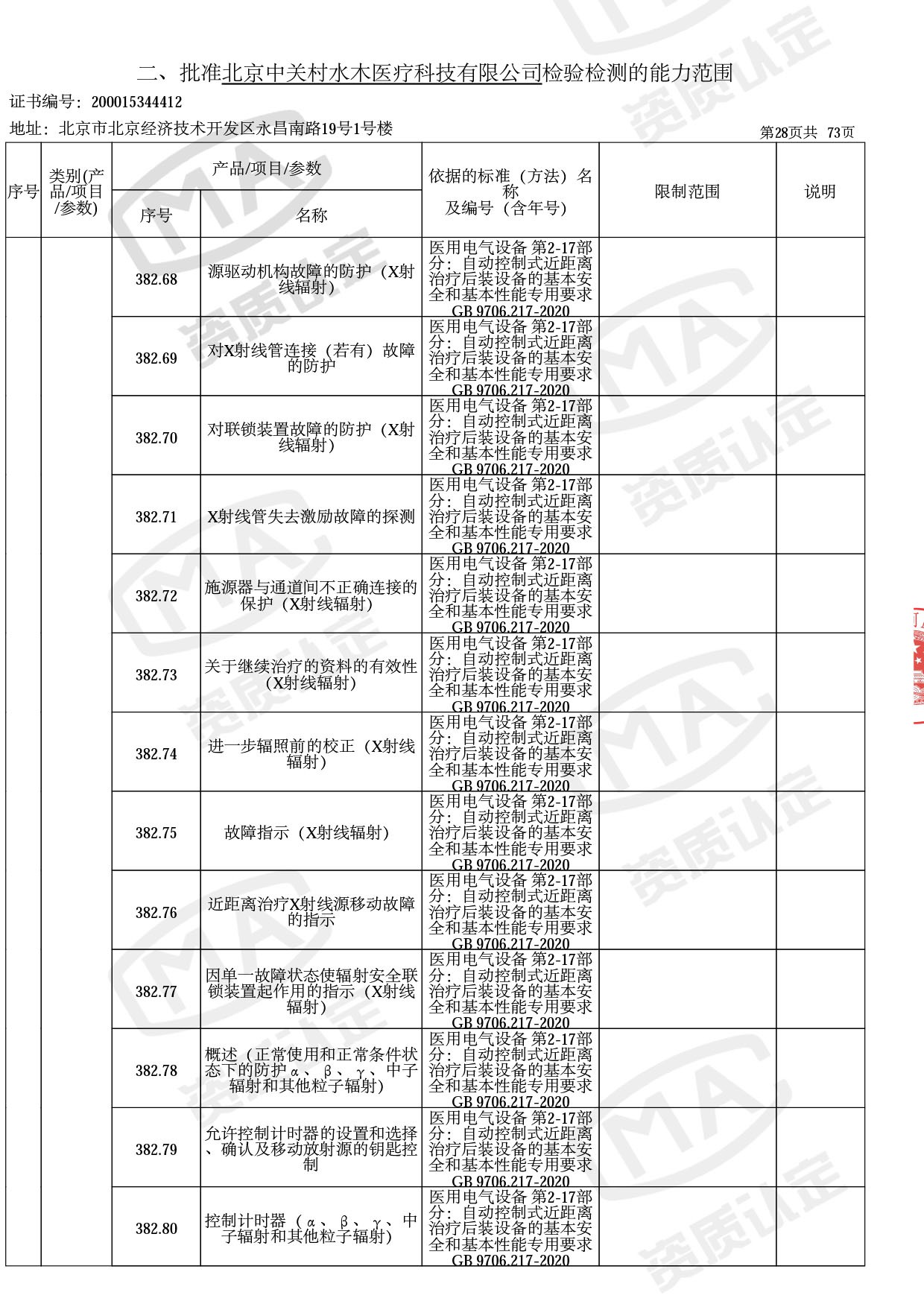
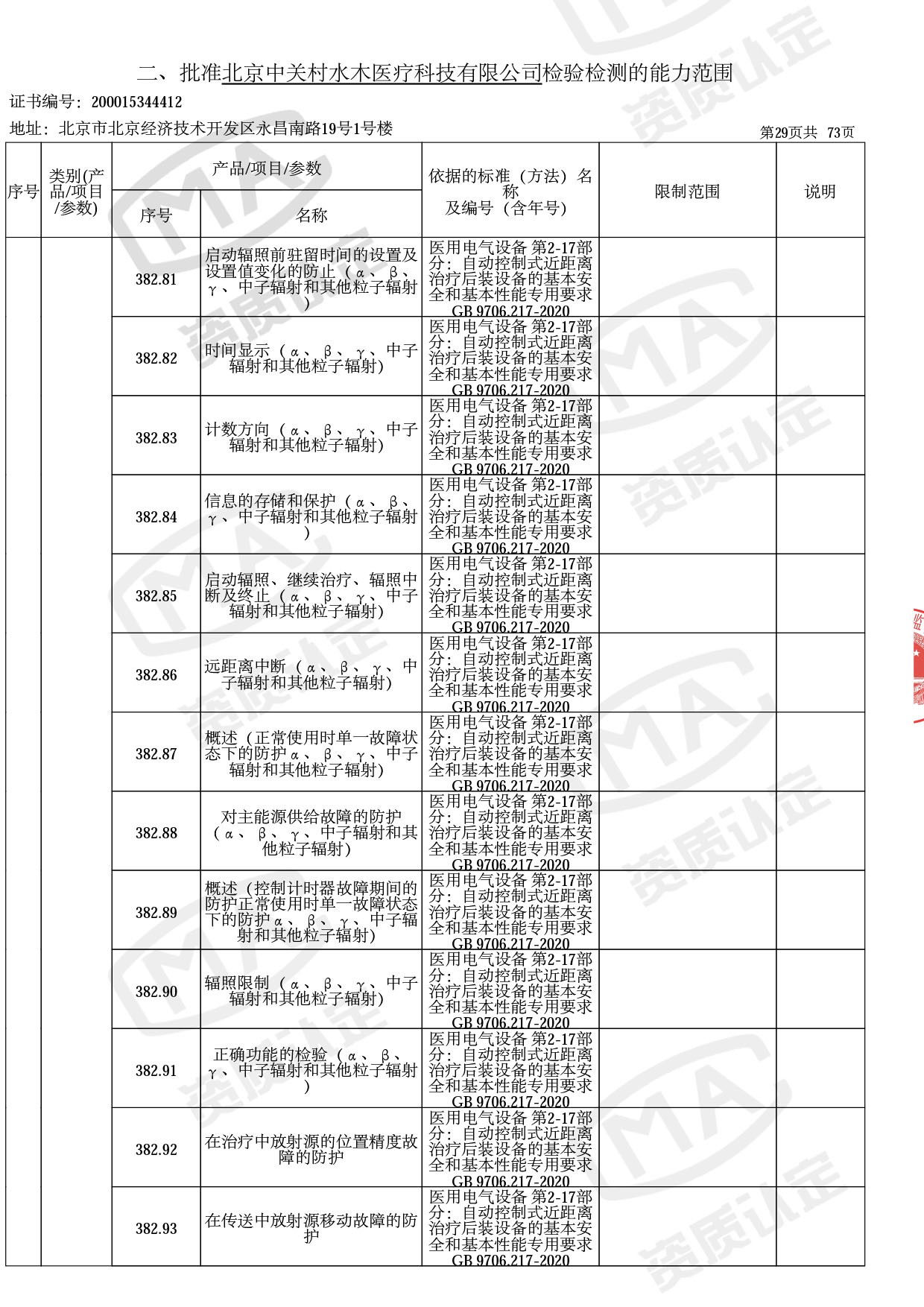
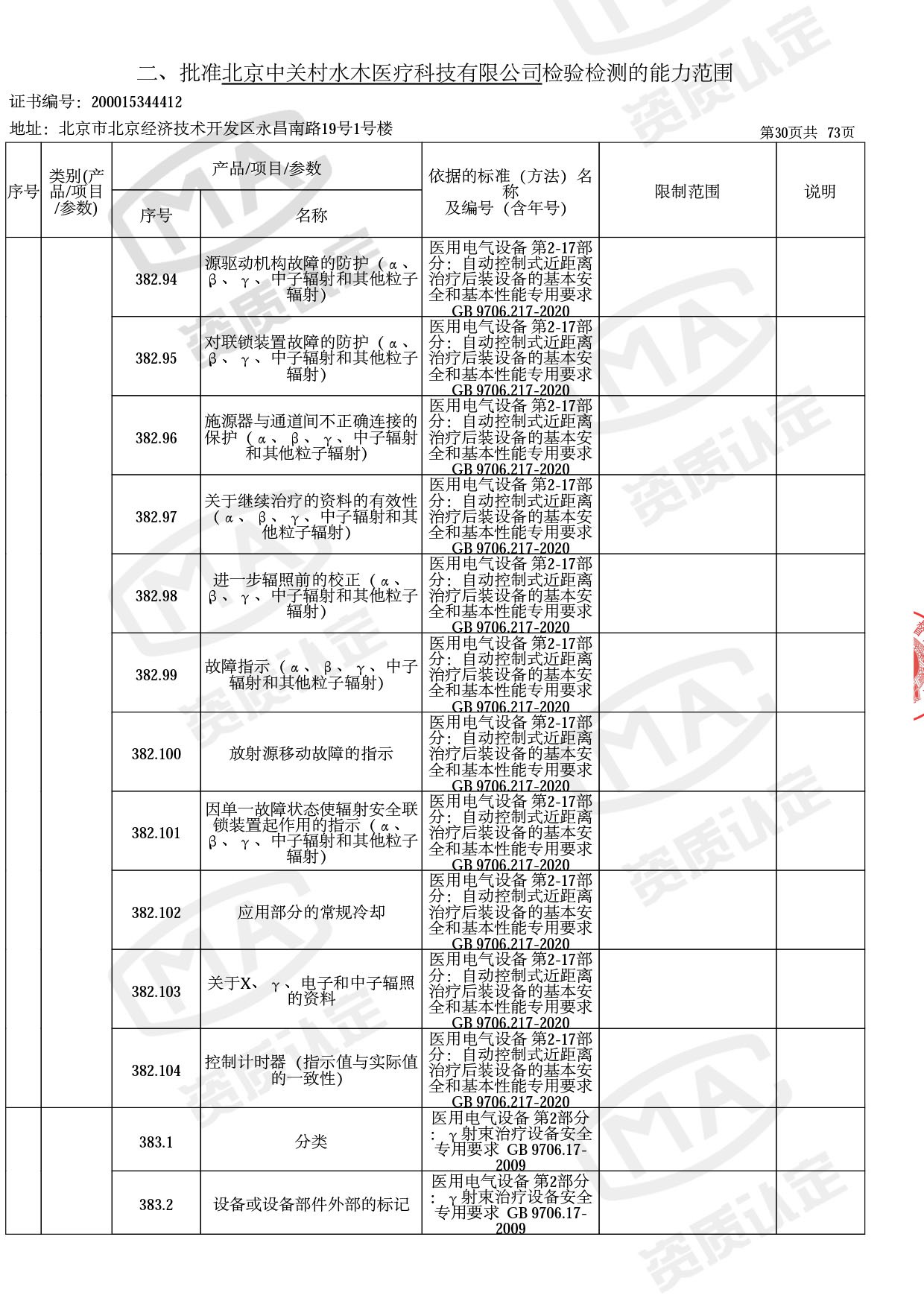
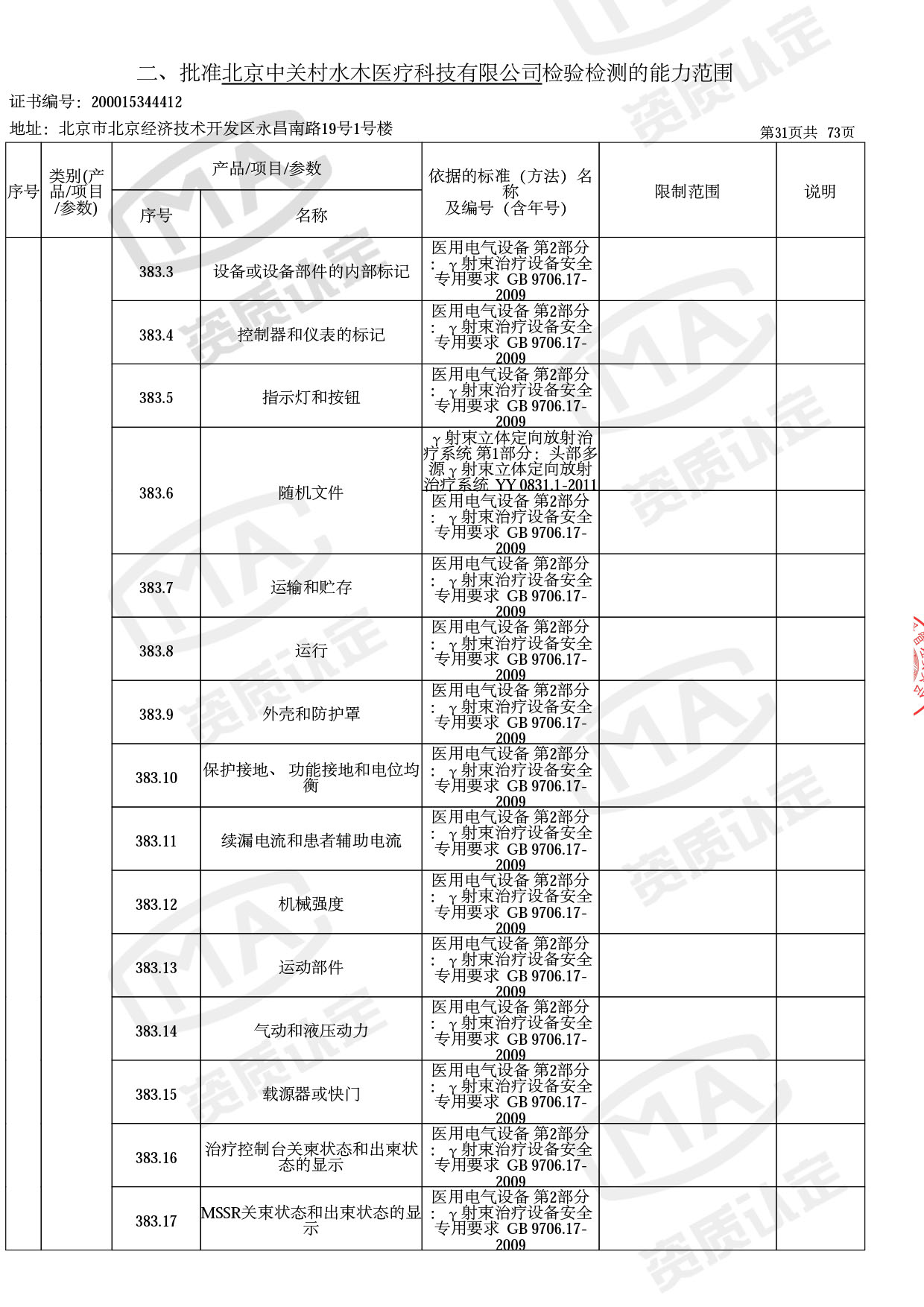
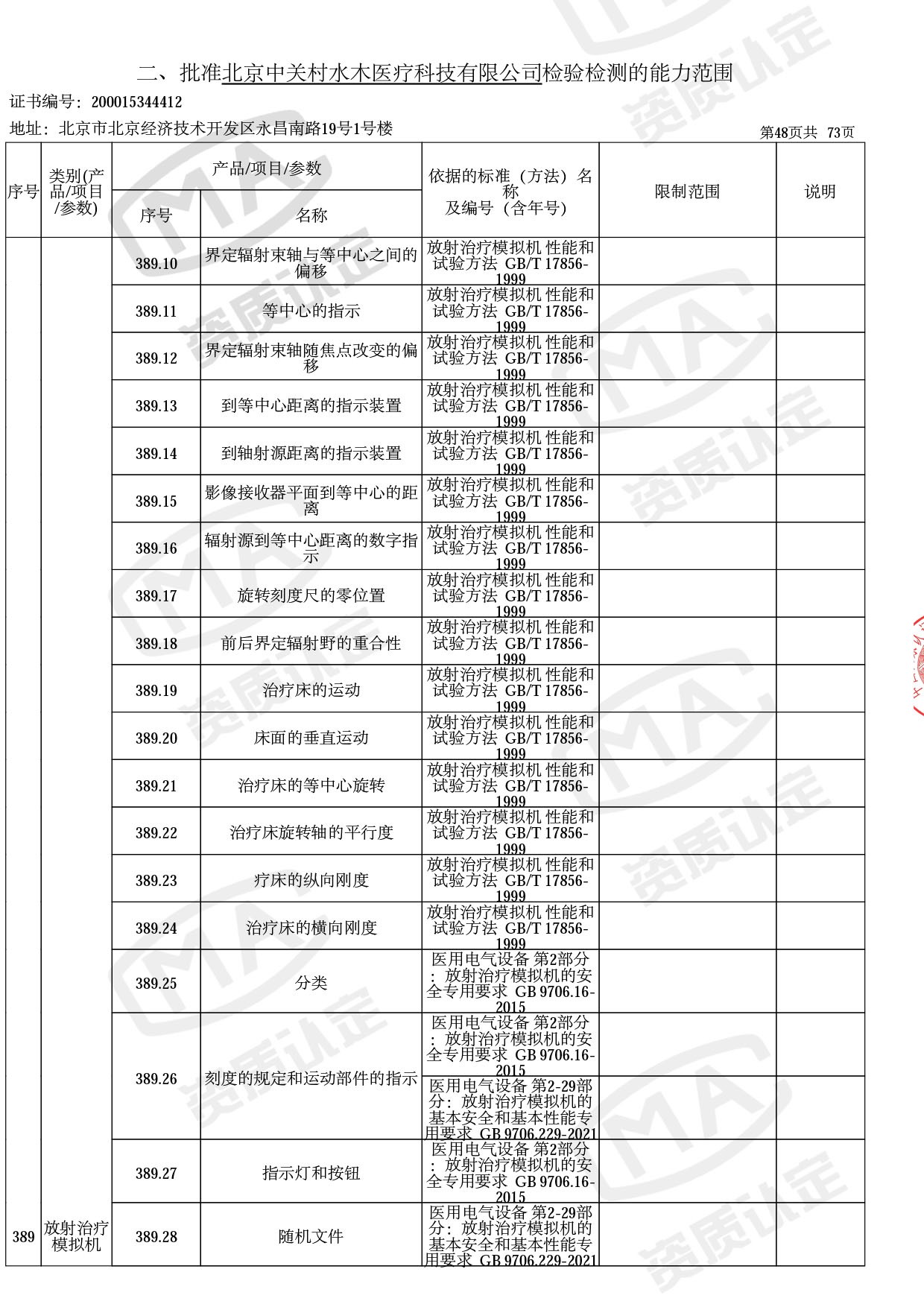
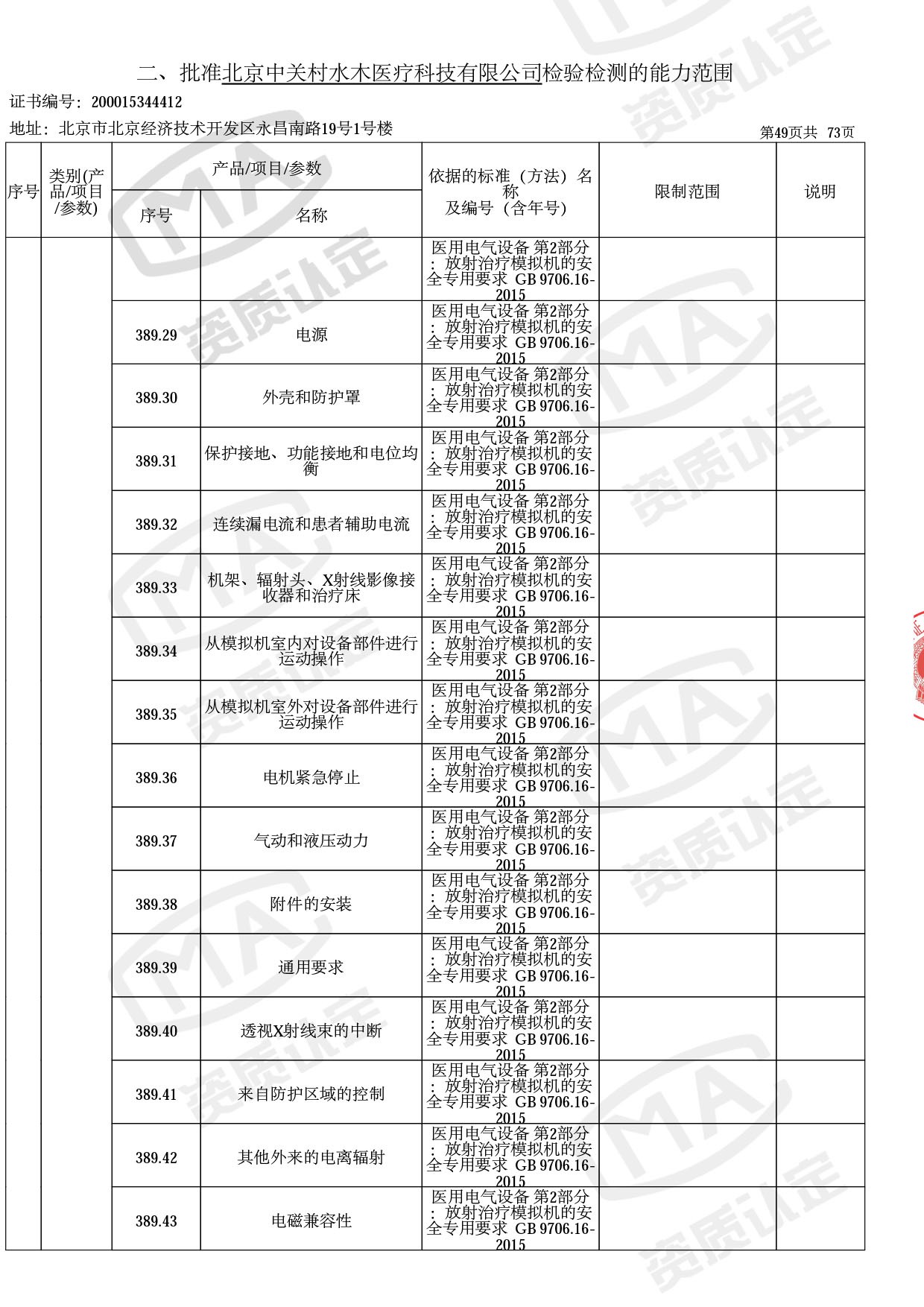
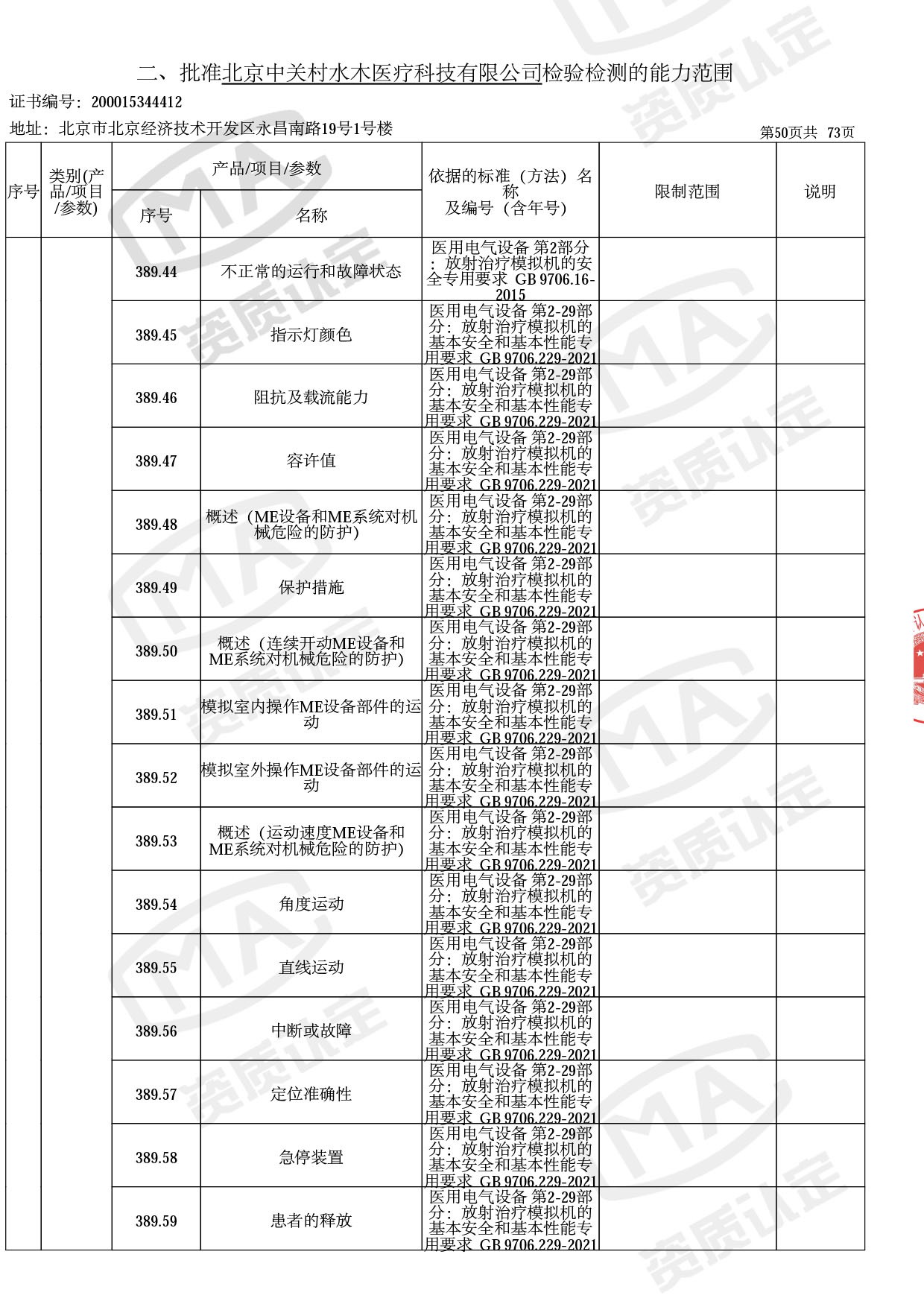
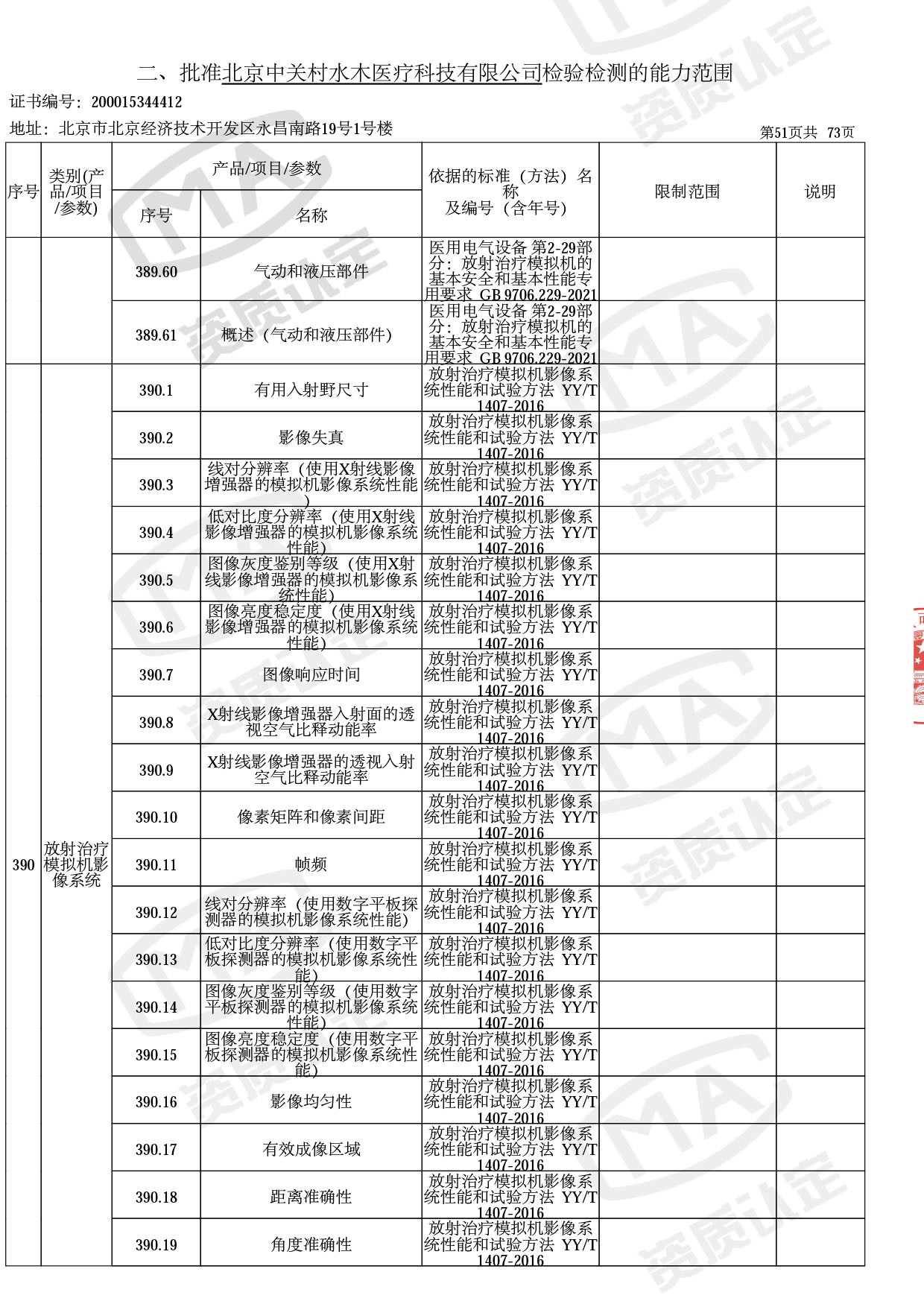
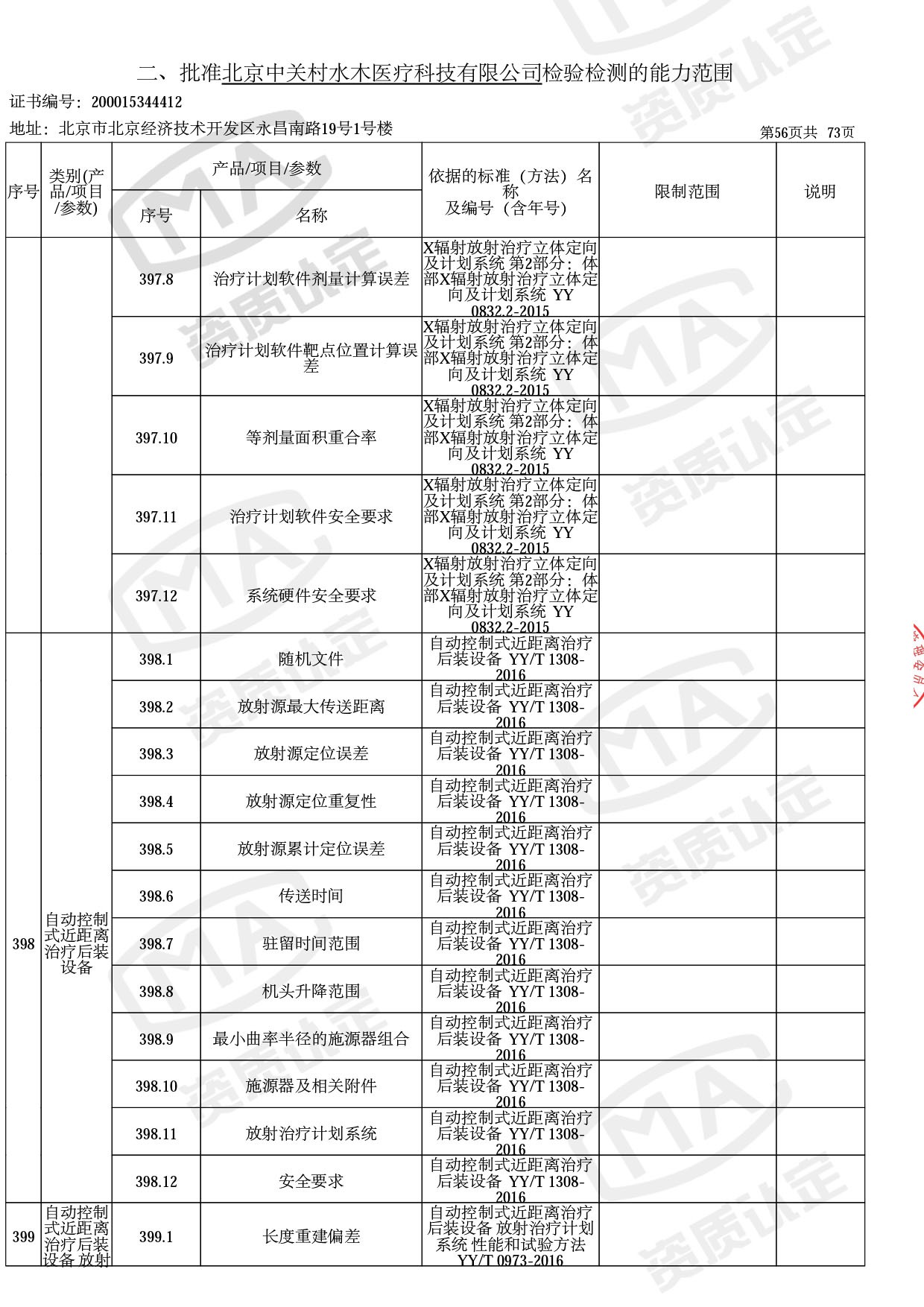
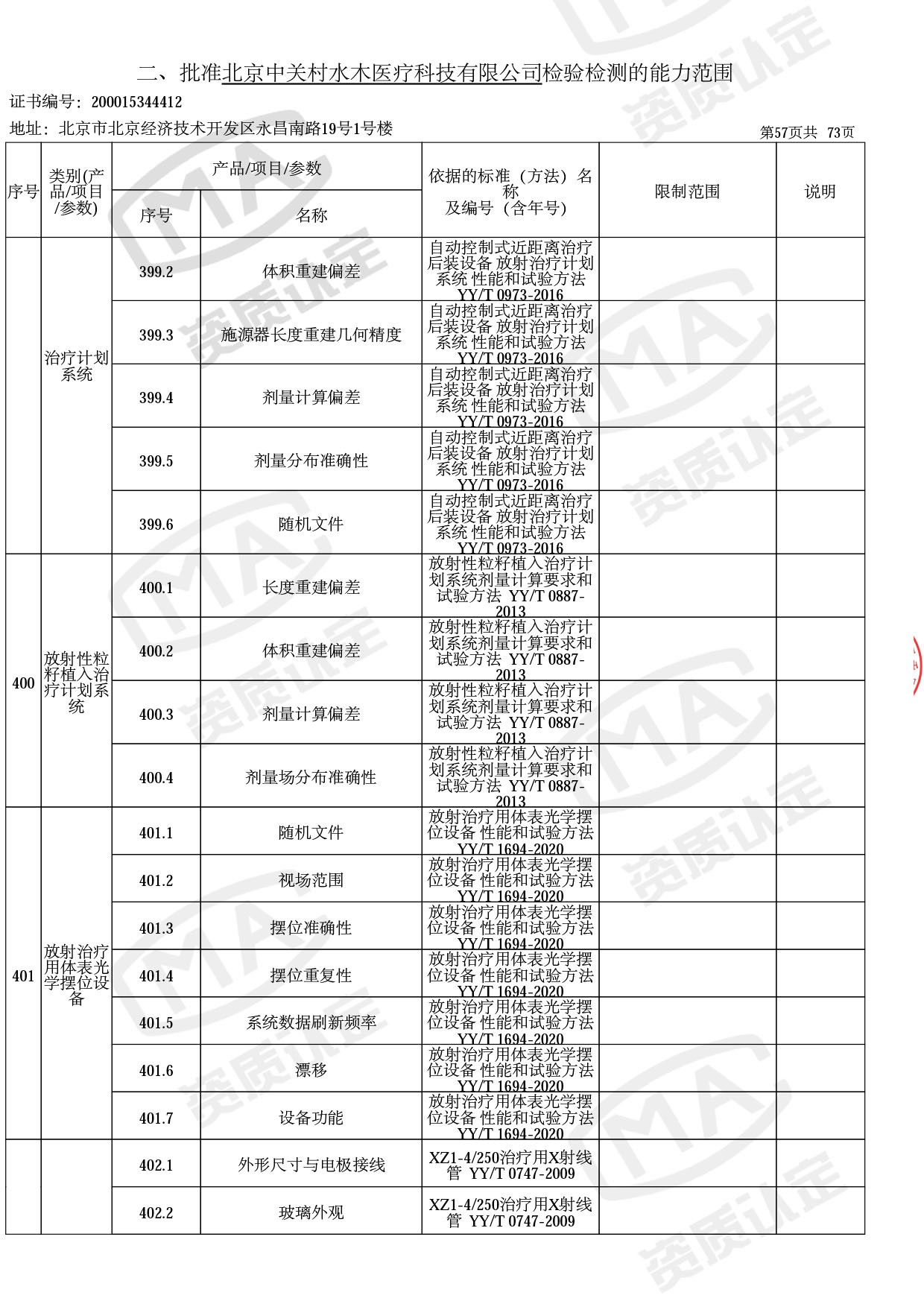
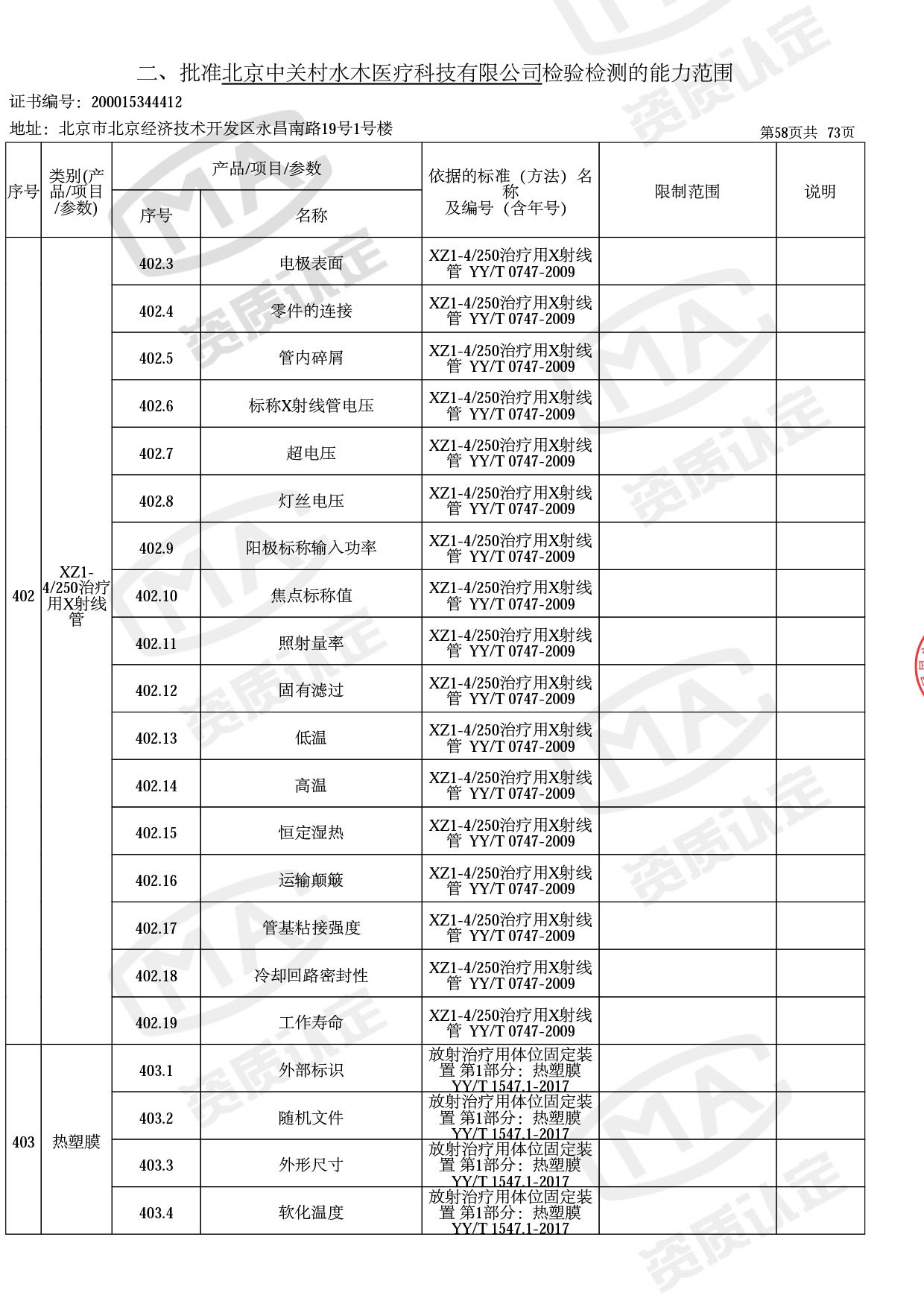
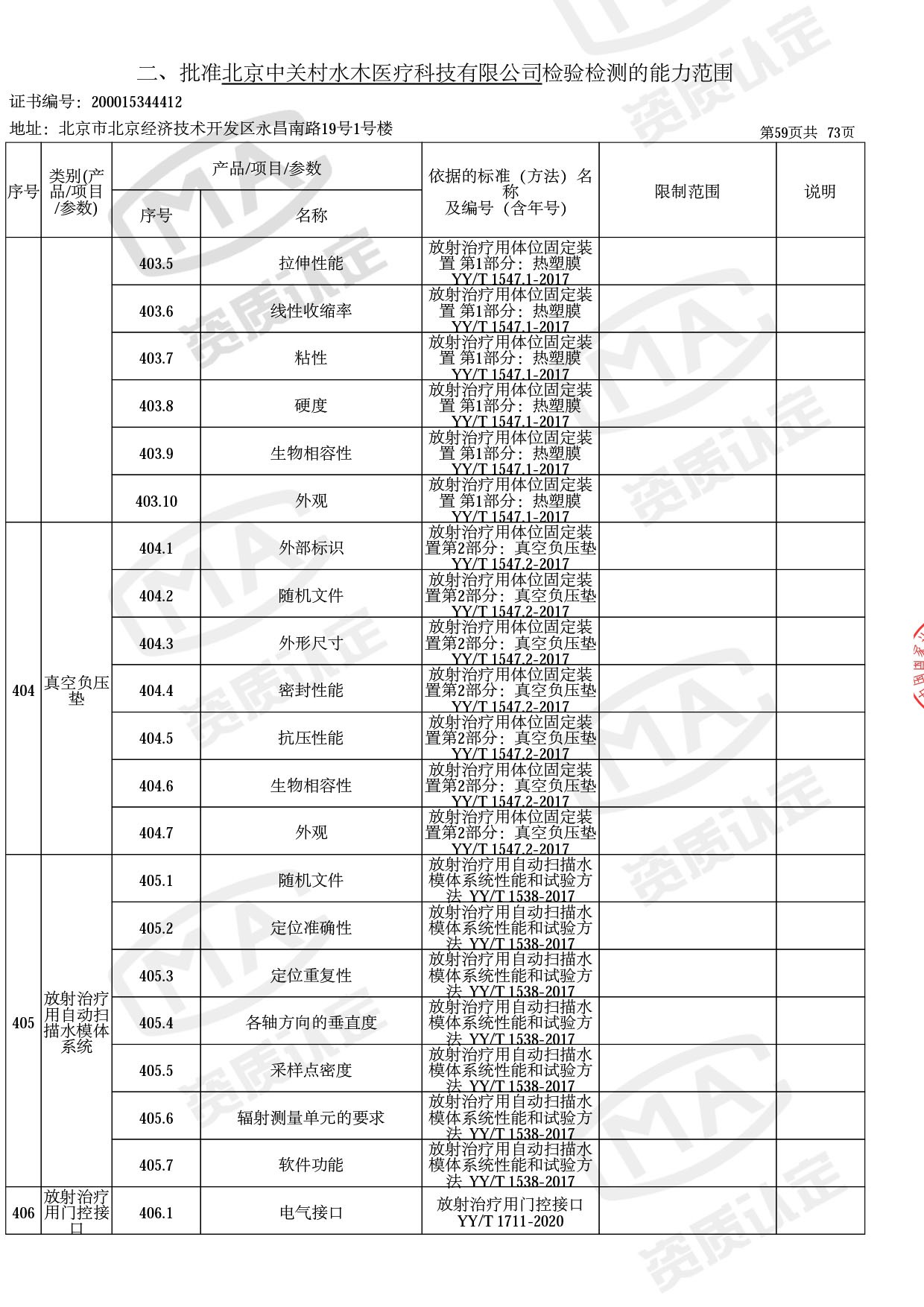
◎ 以下为本次扩项内容 ◎