Zhongguancun Shuimu Medical Technology Service Group
The Zhongguancun Shuimu Medical Technology Service Group was established in 2017 and is headquartered in the Beijing Yizhuang Economic and Technological Development Zone. It is committed to accelerating the process of registration and listing of innovative medical device products, and solving the "last mile" problem of industrialization for innovative medical device companies. The Group is the chairman unit of the Clinical Trial Branch of the China Association for Medical Devices Industry, the executive vice chairman unit of the Application Evaluation Branch of the China Medical Equipment Association, and the chairman unit of the Medical Device Committee of the Beijing Pharmaceutical Association.


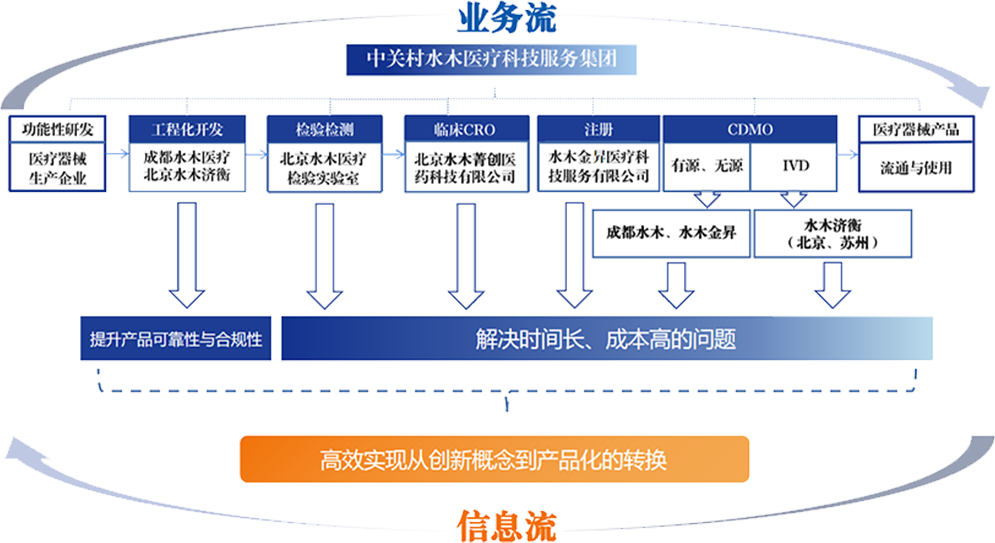
The Group is the world's first to create a "Engineering R&D + Inspection and Testing + Clinical Trials + Registration" one-stop service platform for the entire industry chain. Relying on Beijing Zhongguancun Shuimu Medical Technology Co., Ltd., Beijing Shuimu Jingchuang Pharmaceutical Technology Co., Ltd., Beijing Shuimu Jiheng Biotechnology Co., Ltd., and Chengdu Shuimu Medical Technology Co., Ltd. under the Group, it provides "end-to-end" integrated innovative services for different stages of the entire life cycle of medical device products.
Currently, the Group's business has covered the whole country, and its business development has entered the fast track. As of the end of 2021, the Group has served more than 200 clients, with projects focused on high-end medical devices and in vitro diagnostic products, including the world's smallest proton therapy device, the world's first split medical robot, the country's first heavy ion therapy project, new coronavirus test reagents, in vitro diagnostic reagent standards, and quality control products.
The Group ensures the product registration efficiency of medical device companies through scientific operation processes and strict quality control systems, minimizing review risks and accelerating the pace of product listing.
In September 2021, the State Administration for Market Regulation issued the "Guidance on Further Deepening Reform to Promote the Improvement and Strengthening of the Inspection and Testing Industry", proposing to deepen reform, promote the market-oriented development of inspection and testing institutions, encourage social capital to enter the inspection and testing industry, encourage private enterprises and other social capital to invest in inspection and testing services, support qualified enterprises to apply for relevant qualifications, and provide inspection and testing services to the society.
Under this historical opportunity, the Group will continue to pursue excellence, advocate quality, and have integrity. At the same time, it will take advantage of the momentum, aim at the international technological frontier, further strengthen the technical platform and inspection and testing technology standard system construction, expand capabilities, participate in the design and development of inspection and testing instruments and equipment, reagents and consumables, standard materials, provide "one-stop" services based on quality, achieve "integrated" development, help medical device companies improve R&D innovation and technology iteration efficiency, explore more efficient medical research and production transformation pathways, provide high-quality, efficient, and convenient comprehensive services to the society, and build a one-stop service platform for the industrialization of medical devices.
-
Shuimu Medical Established年
-
Service Platform+
-
Partner Companies+
-
Project Experience+
-
Testing Qualifications+

One-Stop Service Platform for Medical Device Industrialization


Group Advantage Value
-
Policy Dividend
 The state vigorously promotes the development of the medical device industry, and the third-party testing of medical devices is open
The state vigorously promotes the development of the medical device industry, and the third-party testing of medical devices is open -
Unique Model
 We are the world's first to create a one-stop service platform for the medical device industry chain with the unique model of "engineering design + inspection and testing + clinical trials + registration".
We are the world's first to create a one-stop service platform for the medical device industry chain with the unique model of "engineering design + inspection and testing + clinical trials + registration". -
First-mover advantage
 We are the first privately established third-party professional medical device inspection and testing institution in China, and have obtained the China Metrology Certification (CMA) and the China National Accreditation Service for Conformity Assessment (CNAS).
We are the first privately established third-party professional medical device inspection and testing institution in China, and have obtained the China Metrology Certification (CMA) and the China National Accreditation Service for Conformity Assessment (CNAS). -
Our Value
 We shorten the time for obtaining evidence, save customer costs, improve the usability of customer products, help customers quickly obtain evidence, and seize the market.
We shorten the time for obtaining evidence, save customer costs, improve the usability of customer products, help customers quickly obtain evidence, and seize the market. -
Experienced team
 Led by experts from the Ministry of Science and Technology's 13th and 14th Five-Year Plan for Digital Diagnosis and Treatment; core team members are all members of various domestic and international standard committees and industry veterans, having participated in numerous national-level large-scale medical device projects.
Led by experts from the Ministry of Science and Technology's 13th and 14th Five-Year Plan for Digital Diagnosis and Treatment; core team members are all members of various domestic and international standard committees and industry veterans, having participated in numerous national-level large-scale medical device projects.
-
Policy Dividend
 The state vigorously promotes the development of the medical device industry, and the third-party testing of medical devices is open
The state vigorously promotes the development of the medical device industry, and the third-party testing of medical devices is open -
Unique Model
 We are the world's first to create a one-stop service platform for the medical device industry chain with the unique model of "engineering design + inspection and testing + clinical trials + registration".
We are the world's first to create a one-stop service platform for the medical device industry chain with the unique model of "engineering design + inspection and testing + clinical trials + registration". -
First-mover advantage
 We are the first privately established third-party professional medical device inspection and testing institution in China, and have obtained the China Metrology Certification (CMA) and the China National Accreditation Service for Conformity Assessment (CNAS).
We are the first privately established third-party professional medical device inspection and testing institution in China, and have obtained the China Metrology Certification (CMA) and the China National Accreditation Service for Conformity Assessment (CNAS). -
Our Value
 We shorten the time for obtaining evidence, save customer costs, improve the usability of customer products, help customers quickly obtain evidence, and seize the market.
We shorten the time for obtaining evidence, save customer costs, improve the usability of customer products, help customers quickly obtain evidence, and seize the market. -
Experienced team
 Led by experts from the Ministry of Science and Technology's 13th and 14th Five-Year Plan for Digital Diagnosis and Treatment; core team members are all members of various domestic and international standard committees and industry veterans, having participated in numerous national-level large-scale medical device projects.
Led by experts from the Ministry of Science and Technology's 13th and 14th Five-Year Plan for Digital Diagnosis and Treatment; core team members are all members of various domestic and international standard committees and industry veterans, having participated in numerous national-level large-scale medical device projects.