The Zhongguancun Shuimu Medical CDMO platform, independently managed and operated by Beijing Shuimu Jinyi Medical Technology Services Co., Ltd., combines the innovative CRO and CDMO service models to provide customized and integrated services for global medical device manufacturers and clinical institutions. The services cover the entire industry chain process of medical device innovation concepts and technological research, collaborative product development, sample production, research and development transformation, testing and validation, clinical trials, global registration, and supply chain management. This platform reduces the costs for medical device manufacturers to engage different outsourcing service providers, such as re-certification and capacity building, and accelerates the growth of medical device innovation entities.
The company possesses industry-leading project experience, a strong execution team, and rich industry resources. It has established a 9000m² production service platform equipped with high-standard cleanrooms and production areas at the class 10,000 and 100,000 levels, as well as an aseptic verification and testing center. Additionally, the company has a 3500m² research and development laboratory center with specialized laboratories for biochemistry, immunology, PCR, microbiology, and other disciplines.
-
Scientific Research Institute Technology Transfer Project
Medical Device Start-up Project
Imported Medical Device Localization Project
R&D and Production Resource Integration Project
-
Industrial planning
Design transformation
Quality control
Supply chain management
Sample production/mass production
Inspection and detection
clinical trials
Global registration
-
Scientific Research Institute Technology Transfer Project
Medical Device Start-up Project
Imported Medical Device Localization Project
R&D and Production Resource Integration Project
-
Industrial planning
Design transformation
Quality control
Supply chain management
Sample production/mass production
Inspection and detection
clinical trials
Global registration
Service content
research and developmentThe company has a professional software and hardware compliance research and development system of technical team, team members by software and hardware research and development, network security, algorithm research and regulatory compliance personnel. The main members have 15-20 years of work experience in the industry and have experience in artificial intelligence, hardware and software research and development of medical device products, project management and domestic and foreign registration projects.
At present, our team has provided R&D compliance technical services for a number of medical devices and software products, speeding up the enterprise research and development cycle, reducing project costs, and achieving the goal of rapid certification. We can provide comprehensive and efficient compliance technical services for medical device products at all stages of their full life cycle.
-
Product Initiation Stage
- Policy and Regulation Research
- Global Market Access Strategy Planning
- Comparative Product Investigation and Research Outsourcing
- Transformation of Scientific Research Achievements
-
Product Validation and Confirmation Stage
- Registration Inspection of Medical Device Software (including GB/T 25000.51 standard); Network Security Testing and Evaluation (providing a network security report stamped with the CMA seal)
- Vulnerability Scanning/Penetration Testing/Fuzz Testing/Security Feature Testing
- Medical Device Safety Standards/EMC Testing and Rectification
-
Registration and Certification
- Clinical evaluation
- Quality Management System Guidance
- China NMPA registration certification, EU MDR/CE certification, clinical evaluation
- U.S. 510(k) application
- New/Pre-Market Approval
-
Product Development Stage
- Guidelines for Software Development Compliance and Writing of Development Documents
- Implementation of Risk Management Activities and Risk Management Documents
- Guidelines for Network Security Design and Writing of Network Security Documents
- Guidelines for the Construction of Artificial Intelligence Medical Device Database and Writing of Quality Control Documents
-
Usability/Human Factors Engineering
- Human Factors Engineering/Usability Evaluation Plan and Report
- Software Usability Research and Usability Testing Report
-
Specialized Training
- Regulations and standards training
Registration and Clinical Evaluation Services for Domestic and Imported Class II/III Medical Devices, with a Comprehensive Service Management System. Experienced in handling applications for innovative medical devices and medical device classification determination.
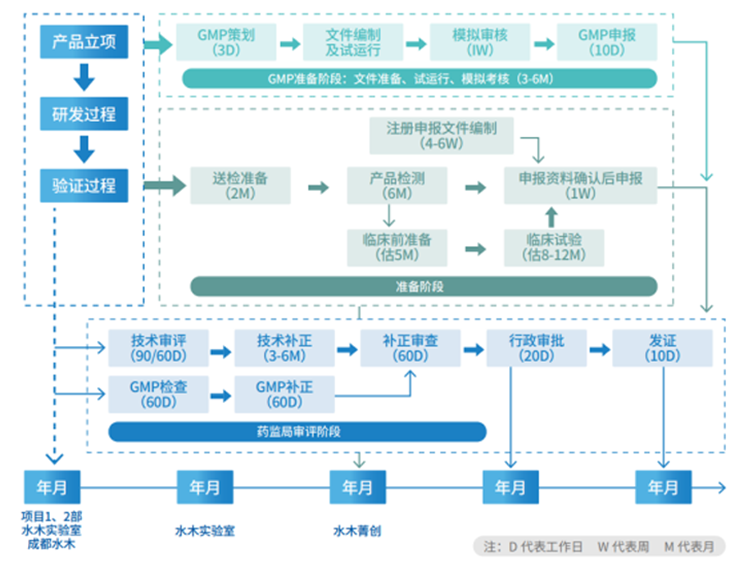
Inspection and Testing Service Acceptance Process
Our company has multiple independent production lines for active/passive/IVD medical devices and a hundred-thousand-level cleanroom, built to create a benchmark quality management system according to ISO3485. We can assist clients in engineering the transformation of prototype models and guide them through a series of validation and rectification processes, enabling rapid achievement of project industrialization goals. Compliance is our top priority, and we uphold high standards to provide reliable and trustworthy contract manufacturing services.
-
project planning
- Product Market Research and Development
- Needs Analysis/Technical Analysis
- Property rights planning
- Research on Industrial Policies and Regulations
-
system construction
- Team Building for Registration and Platform System
- Establishment of Registration System and Platform System
- Registration of Individuals and Platform System Operation
-
R&D commercialization
- Design Requirements/Research and Development Design/Risk Control
- Sample Reagent/Validation
- Material Development, Process Development, and Performance Verification
-
sample production
- Design and Development of Design History File (DHF) Documentation
- System Assessment
- Process Validation
- Small Batch Production
-
inspection and testing
- Electrical safety/EMC/performance testing
- Expiration Date/Transportation Validation
- Software Testing/Network Security/Vulnerability Scanning
- Initial Inspection and Rectification/Reliability Testing/Usability Testing
-
clinical evaluation
- Confirmation of clinical evaluation pathway
- Clinical trial design
- Data statistical analysis
- Clinical evaluation report
-
global registration
- Product classification determination
- Design of registration strategy
- Compilation of registration application documents
- Registration application and regulatory communication and tracking
-
production license
- Production license application
- Verification and rectification of production quality management system
- Production quality control and release
- Cooperation in regulatory/inspection sampling/field inspection/adverse event monitoring, etc
-
mass production
-
project planning
- Product Market Research and Development
- Needs Analysis/Technical Analysis
- Property rights planning
- Research on Industrial Policies and Regulations
-
system construction
- Team Building for Registration and Platform System
- Establishment of Registration System and Platform System
- Registration of Individuals and Platform System Operation
-
R&D commercialization
- Design Requirements/Research and Development Design/Risk Control
- Sample Reagent/Validation
- Material Development, Process Development, and Performance Verification
-
sample production
- Design and Development of Design History File (DHF) Documentation
- System Assessment
- Process Validation
- Small Batch Production
-
inspection and testing
- Electrical safety/EMC/performance testing
- Expiration Date/Transportation Validation
- Software Testing/Network Security/Vulnerability Scanning
- Initial Inspection and Rectification/Reliability Testing/Usability Testing
-
clinical evaluation
- Confirmation of clinical evaluation pathway
- Clinical trial design
- Data statistical analysis
- Clinical evaluation report
-
global registration
- Product classification determination
- Design of registration strategy
- Compilation of registration application documents
- Registration application and regulatory communication and tracking
-
production license
- Production license application
- Verification and rectification of production quality management system
- Production quality control and release
- Cooperation in regulatory/inspection sampling/field inspection/adverse event monitoring, etc
-
mass production
production facilities
service scope
-
Surgical instruments
Active surgical instruments: ultrasonic surgical equipment, laser surgical equipment, high-frequency/radiofrequency surgical equipment, cryo surgical equipment, shock wave surgical equipment, surgical navigation and control systems, endoscopic surgical equipment
-
Medical equipment
Monitoring equipment: comprehensive central monitoring system, electrocardiogram (ECG) diagnostic, electroencephalogram (EEG) diagnostic, blood pressure diagnostic, electromyogram (EMG) diagnostic, respiratory equipment, oxygen concentrator, anesthesia equipment. Therapeutic equipment: electrical therapy, light therapy, physical therapy, magnetic therapy, ultrasound therapy, high-frequency therapy. Blood processing equipment: blood collection equipment, dialysis equipment, cardiopulmonary bypass.
-
Medical consumables
Minimally invasive surgery instruments: puncture needles, suturing devices, endoscopic instruments, etc. Orthopedic implants (metal): bone fixation and replacement implants (plates, screws, pins, rods, bone cement), joint implants, spinal implants. Orthopedic implants (polymer): bone fixation and replacement implants (plates, screws, pins, rods, bone cement). Ophthalmic consumables: artificial lenses, corneal grafts. Dental consumables: dental filling and restoration materials, oral implants, tissue reconstruction materials. Infusion and nursing equipment: injection and puncture devices, infusion pumps, hemostatic instruments, dressings, etc
-
Rehabilitation equipment
Rehabilitation equipment: Orthopedic braces, motion rehabilitation training equipment
-
Traditional Chinese medicine equipment
Traditional Chinese medicine equipment: Traditional Chinese medicine diagnostic equipment, traditional Chinese medicine treatment equipment.
-
Home care
Wearable devices: Rehabilitation robot orthopedic braces. Low-end home care: Blood glucose meters, blood pressure monitors, thermometers.
-
In vitro diagnostic (IVD) reagents
Molecular diagnostic reagents: PCR reagents, FISH reagents, NGS reagents. Immunoassay diagnostic reagents: Luminometric immunoassay reagents, chemiluminescence reagents, fluorescent immunoassay reagents, immunoblotting reagents, etc. Blood and body fluid analysis: Blood cell analysis, coagulation analysis, erythrocyte sedimentation rate analysis, flow cytometry analysis. Immunochromatographic reagents: Colloidal gold test strips, immunofluorescence test strips, quantum dot immunochromatography. Others: General chemical reagents.
-
Medical devices
Clinical diagnostic instruments: sequencers, chemiluminescence analyzers, flow cytometers, PCR machines, fluorescence immunoassay analyzers