Company Overview
The Zhongguancun Shuimu Medical Technology Service Group was established in 2017 and is headquartered in the Beijing Yizhuang Economic and Technological Development Zone. It is committed to accelerating the process of registration and listing of innovative medical device products, and solving the "last mile" problem of industrialization for innovative medical device companies. The Group is the chairman unit of the Clinical Trial Branch of the China Association for Medical Devices Industry, the executive vice chairman unit of the Application Evaluation Branch of the China Medical Equipment Association, and the chairman unit of the Medical Device Committee of the Beijing Pharmaceutical Association.


Development History
Shuimu Medical is a rapidly growing company. While continuously improving our global layout and increasing the number of employees, we are also expanding our professional technology and platform capabilities in multiple fields to meet the unmet needs of patients.




Subsidiary
-
Chengdu Shuimu Medical Technology Co., Ltd.
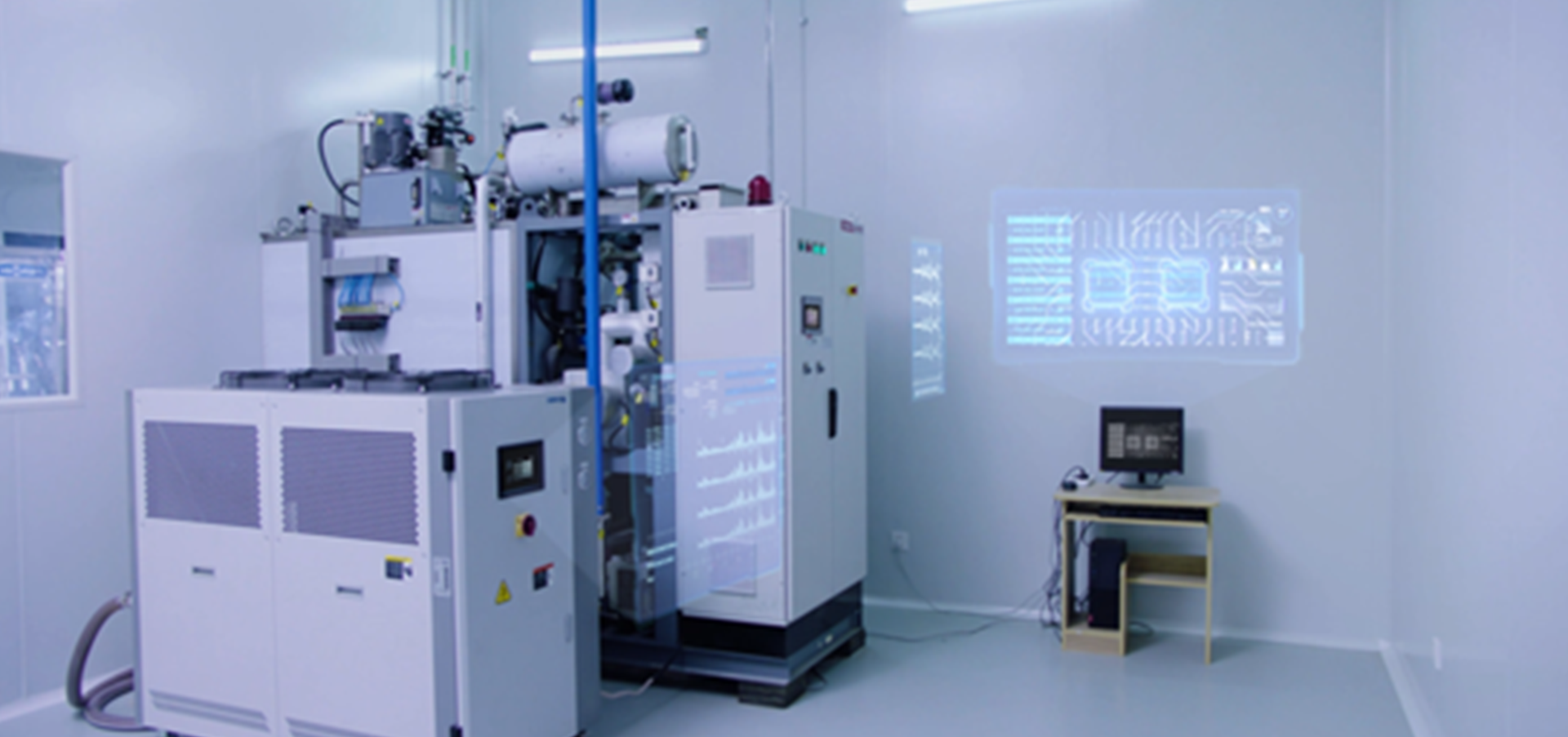
Device Design and Evaluation Platformreliability analysis
Device evaluation
Intelligent fault warning
Remaining life prediction
Validity period evaluation
software development
TEL:028-61417786
-
Beijing Shuimu Jingchuang Pharmaceutical Technology Co., Ltd.
Clinical CRO PlatformClinical Trial Research
Global Registration
Risk Management
Clinical Trial Quality Management
Project Management/Clinical Monitoring
Medical Writing/Biostatistics/Data Management
Platform Integration
TEL:010-87766630
-
Beijing Shuimujiheng Biotechnology Co., Ltd
IVD standard and quality control product research and development production platformThird-party Quality Control Products
Reference Material Customization
CDMO Services
TEL:010-59771556
-
Beijing Shuimu Jinsheng Medical Technology Service Co., Ltd.
CDMO platformRadiation Therapy Physics Quality Control
Hospital Oncology Radiotherapy Department Construction
Radiation Therapy Patient Recruitment
TEL:18612116215
-
Beijing Shuimulingrui Medical Technology Co., Ltd.
Digital Operation Platform for Life Science IndustryIndustry Planning
Design Transformation
Quality Management
Supply Chain Management
Sample Production/ Mass Production
Inspection and Testing
Clinical Trials
Global Registration
TEL:13522767962


Team Introduction
Management Team - Meet our experienced and innovative management team


Corporate News
-
14
2023-09
《医疗器械创新论坛》园区行【南京站】成功举办
9月7日,由中国生物工程学会指导,火石数链主办的“CBIB园区行”医疗器械专场活动,在南京江北生物医药谷成功举办。活动邀请了江苏省医疗器械检验所领导、多家医疗器械头部企业、 医疗器械创新产业链服务商共聚交流,吸引了80余人前来参会,共同交流,探讨器械研发生产问题,助力提升园区及周边企业研发创新实力和效率.9月7日,由中国生物工程学会指导,火石数链主办的“CBIB园区行”医疗器械专场活动

-
01
2023-11
CMEF盛会盘点!水木金昇总经理冯健主讲《软件网络安全开发过程及安全能力评估》
2023年10月28日—31日,由中国食品药品企业质量安全促进会医疗器械分会联合国药励展展览有限责任公司主办全球医疗器械产业“航母级”盛会——第88届CMEF中国国际医疗器械(秋季)博览会(下文简称“CMEF博览会”)在深圳正式举办。

-
03
2023-11
喜报!中关村水木医疗再获国家级奖项!打造“校企合作”新范式
近日,中国高等教育学会公布了2022年度中国高等教育学会“校企合作 双百计划”典型案例名单,由北京中关村水木医疗科技有限公司(以下简称“中关村水木”)作为申报单位、电子科技大学作为合作单位申报的“医疗器械辅助研发提升-可靠性创新平台”项目被认定为2022年度中国高等教育学会“校企合作 双百计划”典型案例。

-
06
2023-12
第三次扩项!中关村水木医疗顺利通过“放疗、网络安全”领域扩项现场评审
中关村水木医疗团队在行业内深耕多年,丰富的专业经验能够为国外企业进入中国市场提供从本土化研发到注册生产的全流程技术服务,同时也能够助力国内企业走出国门走向国际化。