Beijing Shuimu JiHeng Biotechnology Co., Ltd
IVD standard and quality control product research and production platformBeijing Shuimu JiHeng Biotechnology Co., Ltd. is a comprehensive service provider of domestically developed standard products in all categories. Its research and development laboratory covers an area of 1000m², and its GMP production workshop covers an area of 1300m².
The company provides comprehensive services for the entire process of research, registration, regulation, and use of in vitro diagnostic products. It focuses on the research and development of in vitro diagnostic reagent standards and quality control products. It is a high-tech enterprise with core advantages in product research, production, and service resources.
-
Enterprise/Research Institution
Technical consulting
CDMO
End-to-end quality control program
-
Clinical Laboratory/Medical Examination Center
Quality control
Quality management
ISO15189 accredited advisory services

-
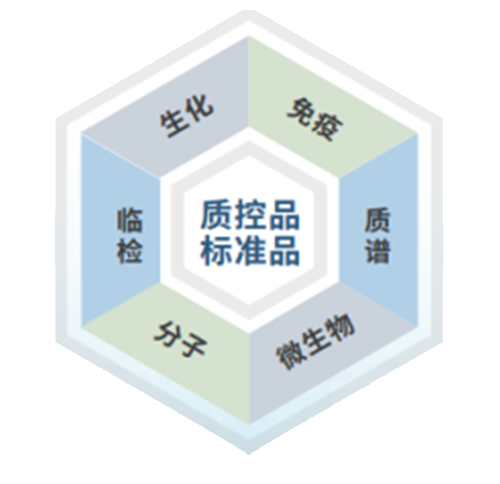
Control and Standard Products
Technical consulting
CDMO
End-to-end quality control program
-
企业/Clinical Laboratory/Medical Examination Center
Quality control
Quality management
ISO15189 accredited advisory services
Service Content
-
Biochemical technology platform
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
-
Biochemical technology platform
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
-
Biochemical technology platform
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
-
Biochemical technology platform
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
-
Biochemical technology platform
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
-
Biochemical technology platform
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection
Pancreatitis detection