Inspection and Testing Service Platform
Beijing Zhongguancun Shuimu Medical Technology Co., Ltd.Beijing Zhongguancun Shumu Medical Technology Co., Ltd. is the first privately-owned third-party professional medical device inspection and testing organization established independently in China. It has obtained the China Metrology Accreditation (CMA) and the China National Accreditation Service for Conformity Assessment (CNAS) certifications. It is also one of the first CMA and CNAS certified organizations to obtain testing capabilities for the general requirements of medical electrical equipment according to GB 9706.1-2020 standard.
Laboratory Overview
The company has specialized testing laboratories and a mature R&D team, with a laboratory space of 3500 square meters. The business scope mainly covers active medical devices and in vitro diagnostic reagents, with medical device electromagnetic compatibility testing laboratory, electrical safety testing laboratory, reliability testing laboratory, packaging testing laboratory, software testing laboratory, and IVD testing laboratory.
-

霉菌试验箱
-

门框试验装置
-

模拟运输台 斜面冲击试验台
-

耐压测试仪
-

内窥镜实验室
-

耦合器传声器
-

屏蔽室
-

球压试验装置
-

燃烧试验机
-

三综合试验系统(温度、湿度、振动)
-

生化免疫实验室
-

生理信号模拟器
-

湿化器测试工装
-

示波器、峰值断电开关装置
-

输液泵分析仪
-

数字电参数测量仪
-

太阳辐射试验箱
-

微波漏能测试仪
-

维卡软化试验机
-

温度冲击试验箱
-

温度低气压综合试验箱
-

物料间
-

压力堆码试验台
-

医用电子及康复理疗实验室
-

振动试验台
-

整改台
-

整改用屏蔽室
-

制备间
-

制冷恒温槽
-

质谱实验室
-

中性盐雾腐蚀箱
-

主被动模拟肺测试工装
-

3米法半电波暗室
-

10m³步入式高低温湿热试验箱
-

10米法半电波暗室
-

10米法半电波暗室外观
-

30m³步入式高低温湿热试验箱
-
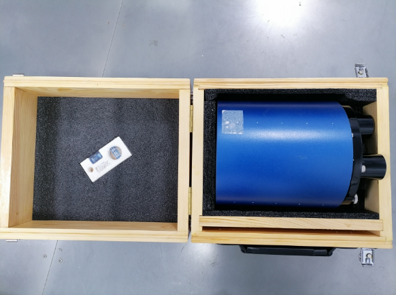
CT 性能测量模体
-
DQE量子探测效率检测仪
-

Fonix8000助听器测试系统
-

HA-1型耦合器
-

HA-2型耦合器
-

PCR实验室
-

X射线、CT实验室
-

X射线质量分析仪
-

按压开关寿命试验机
-

暗室控制室
-

把手提拎试验装置
-
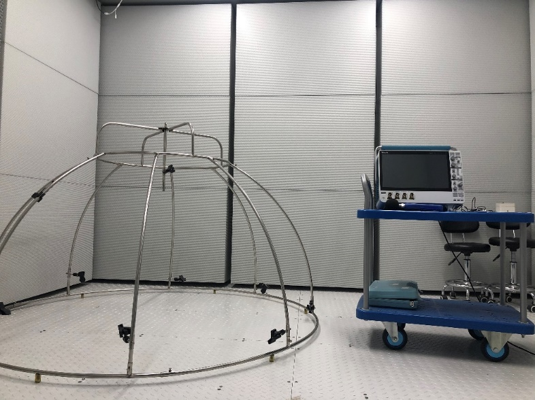
半消音实验室
-

变压器感应耐压测试仪
-

便携油槽
-

表面测温体模、仿组织体模、切片厚度体模
-

超声功率计
-

超声检测实验室
-

超声声输出测量系统
-
成像性能测试模体
-

电刀分析仪
-

电气安全测试仪
-

电气整改元件库
-

电学实验室
-

电源线拉扭试验机
-

跌落试验台
-

防尘试验箱
-

防水试验装置
-

高加速冲击碰撞台
-

鼓风干燥箱
-

光生安全实验室
-

光束质量分析仪
-

呼吸机、麻醉机测试工装
-

呼吸麻醉实验室
-

机械实验室
-

激光功率计
-

激光实验室
-

结构材料及温度实验室
-

精密模拟肺
-

抗扰度实验室
-

酶学实验室
Service Content
-

Business Display Business Display Business Display
Business Display Business Display Business Display
-

Business Display Business Display Business Display
Business Display Business Display Business Display
-

Business Display Business Display Business Display
Business Display Business Display Business Display
-

Business Display Business Display Business Display
Business Display Business Display Business Display
-

Business Display Business Display Business Display
Business Display Business Display Business Display
-

Business Display Business Display Business Display
Business Display Business Display Business Display
-

Business Display Business Display Business Display
Business Display Business Display Business Display
-

Business Display Business Display Business Display
Business Display Business Display Business Display
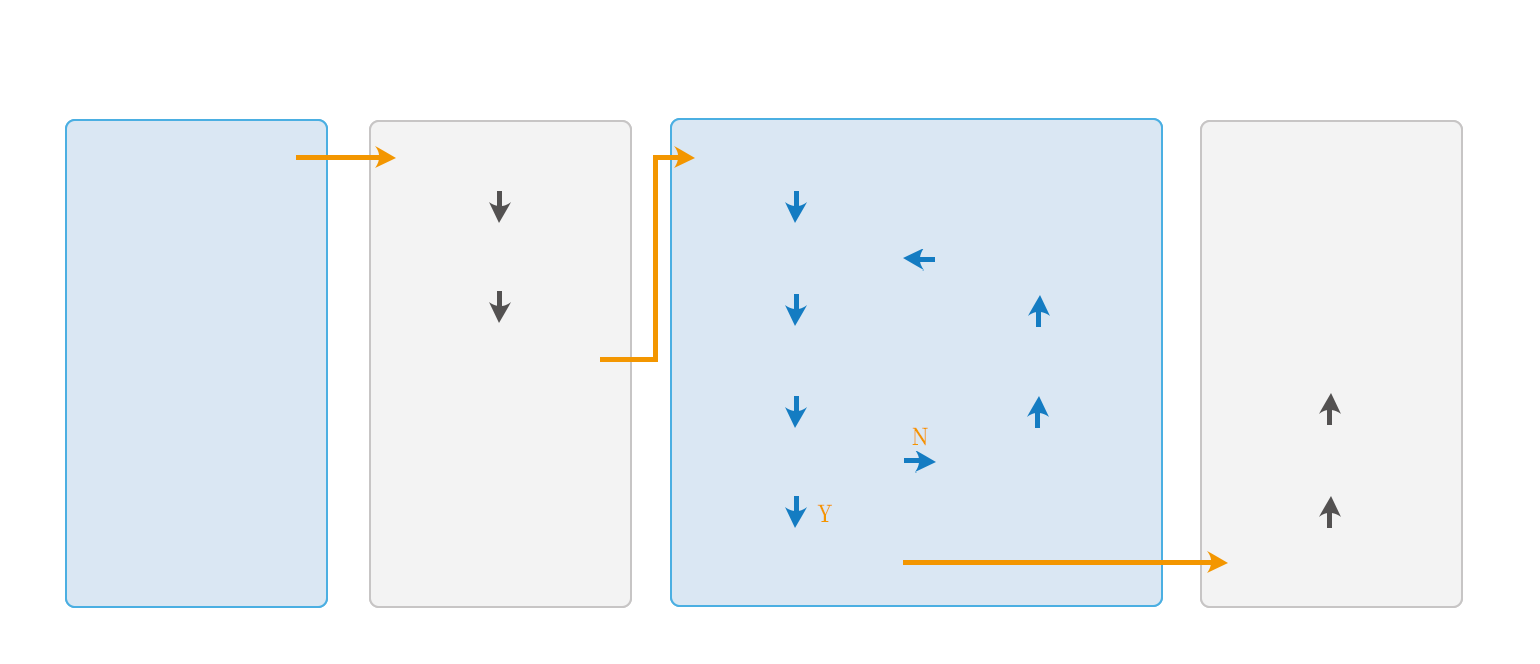
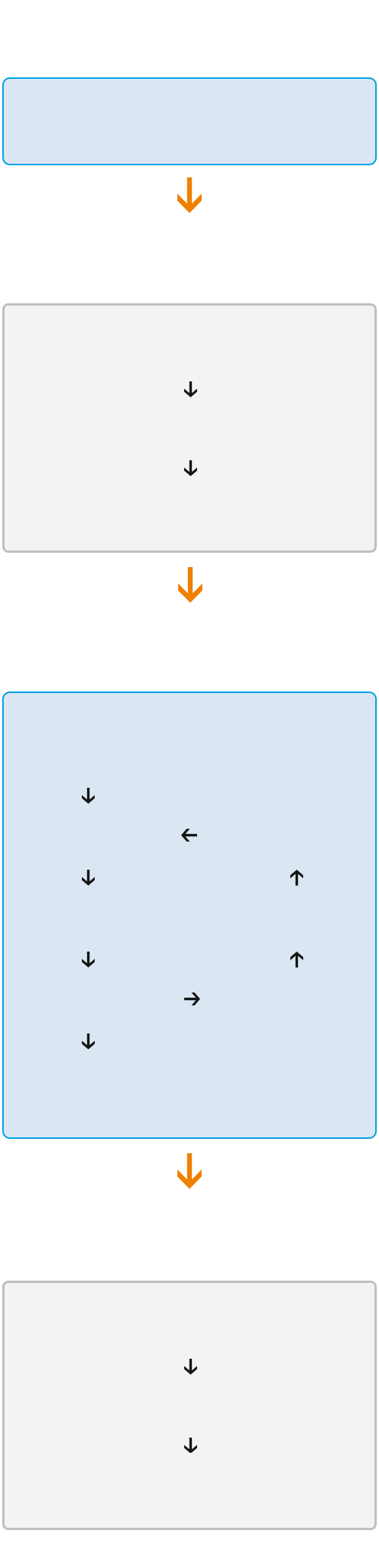
Inspection and testing service acceptance process
-
Consultation Stage
Business Consultation
-
Intention Agreement Stage
Intention Agreement
Business Evaluation
Quotation Confirmation
-
Testing Phase
Contract Signing
Determine test plan
Execute test
Result evaluation test
Issue test report
Rectification plan
Evaluation equipment
Rectification effect
-
Delivery phase
Project completion
Expense recognition
Service content confirmation
-
Consultation Stage
Business Consultation
-
Intention Agreement Stage
Intention Agreement
Business Evaluation
Quotation Confirmation
-
Testing Phase
Contract Signing
Determine test plan
Execute test
Result evaluation test
Issue test report
Rectification effect
Evaluation equipment
Rectification plan
-
Delivery phase
Project completion
Expense recognition
Service content confirmation
Medical Device Testing Standards


Inspection and testing service platform
Contact number: 010-67867683 website: www.zecsm.cn Office address: Building 1, No. 19 South Yongchang Road, Economic and Technological Development Zone, Beijing