Beijing Zhongguancun Shuimu Medical Technology Co., Ltd.
Inspection and Testing Service Platform 最全面的检测范围 最完善的检测体系-

-
Building 1, No. 19 Yongchang South Road, Beijing Economic and Technological Development Zone
East China | South China | Northeast | Northwest: 18610900675/18610907598
North China | Central China | Southwest | Hong Kong, Macao, : 17801120658/18610900670

Beijing Zhongguancun Shuimu Medical Technology Co., Ltd. 国内首家自主建立的第三方专业医疗器械检验检测机构
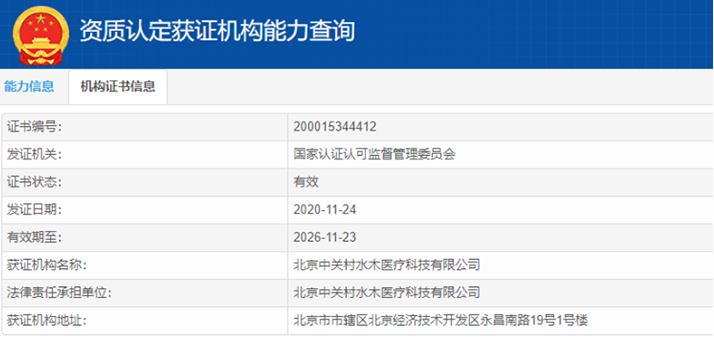






Honors/Certificates
-

检验检测机构资质认定证书
-

中国合格评定国家认可委员会实验室认可证书
-

ISTA证书
-

检验检测机构资质认定证书

-

Electrical Safety Laboratory
-

Electromagnetic Compatibility Laboratory
-

Respiratory Anesthesia Laboratory
-
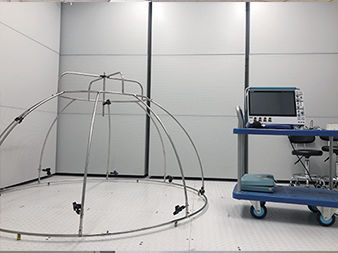
Semi-anechoic Laboratory
-

Reliability Laboratory
-

Mechanical Laboratory
-

Software Testing Laboratory
-

IVD Mass Spectrometry Laboratory